The application of RNA is not just limited to vaccines; it also holds the potential to play a considerable role in diagnostics, therapeutics and research. Let’s take a look at the future of RNA applications.
Future of RNA in diagnostics
mRNA bridges the gap between DNA and proteins. Apart from mRNA, the cell contains other types of RNA molecules that play regulatory roles. These other types of RNA molecules include long non-coding RNAs (lncRNA), small interfering RNAs (siRNA), and microRNAs (miRNA). It has been reported that tumour cells release extracellular vesicles containing stable RNA molecules into the bloodstream. This RNA in the bloodstream can serve as a non-invasive biomarker for cancer diagnosis.
Early diagnosis of cancer is crucial in driving an effective treatment plan. Cholangiocarcinoma (CCA), for instance, is a malignant tumour that is commonly found in the bile duct. Unfortunately, the predicted treatment outcomes for CCA are generally poor. Therefore, researchers have studied the RNA sequences and miRNA profiles of CCA patients and specifically identified 13 mRNAs, 26 lncRNAs and 3 miRNAs. The expression level of some of these RNA species correlates with clinical features including vascular invasion, pathologic stage and neoplasm histologic grade. It was concluded that these lncRNAs, miRNAs and mRNAs can serve as prognostic and diagnostic biomarkers, as well as therapeutic targets for patients with CCA (Cho, Chan, & Wong, 2020; Long et al., 2019).

In addition to cancer, RNA can serve as a diagnostic biomarker for other diseases. For instance, a high level of programmed death ligand 1 (PD-L1) mRNA is detected in the saliva of periodontitis (inflammatory condition of the tissue that surrounds and supports the teeth) patients. PD-L1 is involved in causing inflammation. The measurement of PD-L1 mRNA level in saliva can serve as a parameter to distinguish periodontitis patients from healthy individuals and can also be used to assess the severity of the disease (Cho et al., 2020; Yu et al., 2019).
Going one step further from diagnostics, what is the future of RNA in therapeutics?
Future of RNA as therapeutically agent
RNA therapies have emerged quite recently. However, various RNA therapies have already made it into clinics. RNA drugs have been approved for amyloidosis (accumulation of the protein amyloid in nerves and organs), Duchenne muscular dystrophy (inherited muscle degeneration disorder) and spinal muscular atrophy (genetic neuromuscular condition). Here, RNA-based therapies alter how the message encoded by a mutated gene is produced, modified or degraded. This way, RNA drugs either restore the amount of produced mRNA to a ‘normal’ level, or alter the transcribed mRNA molecule in such a way that it mimics the mRNA molecule that is transcribed from the non-mutated WT gene (Harries, 2019).
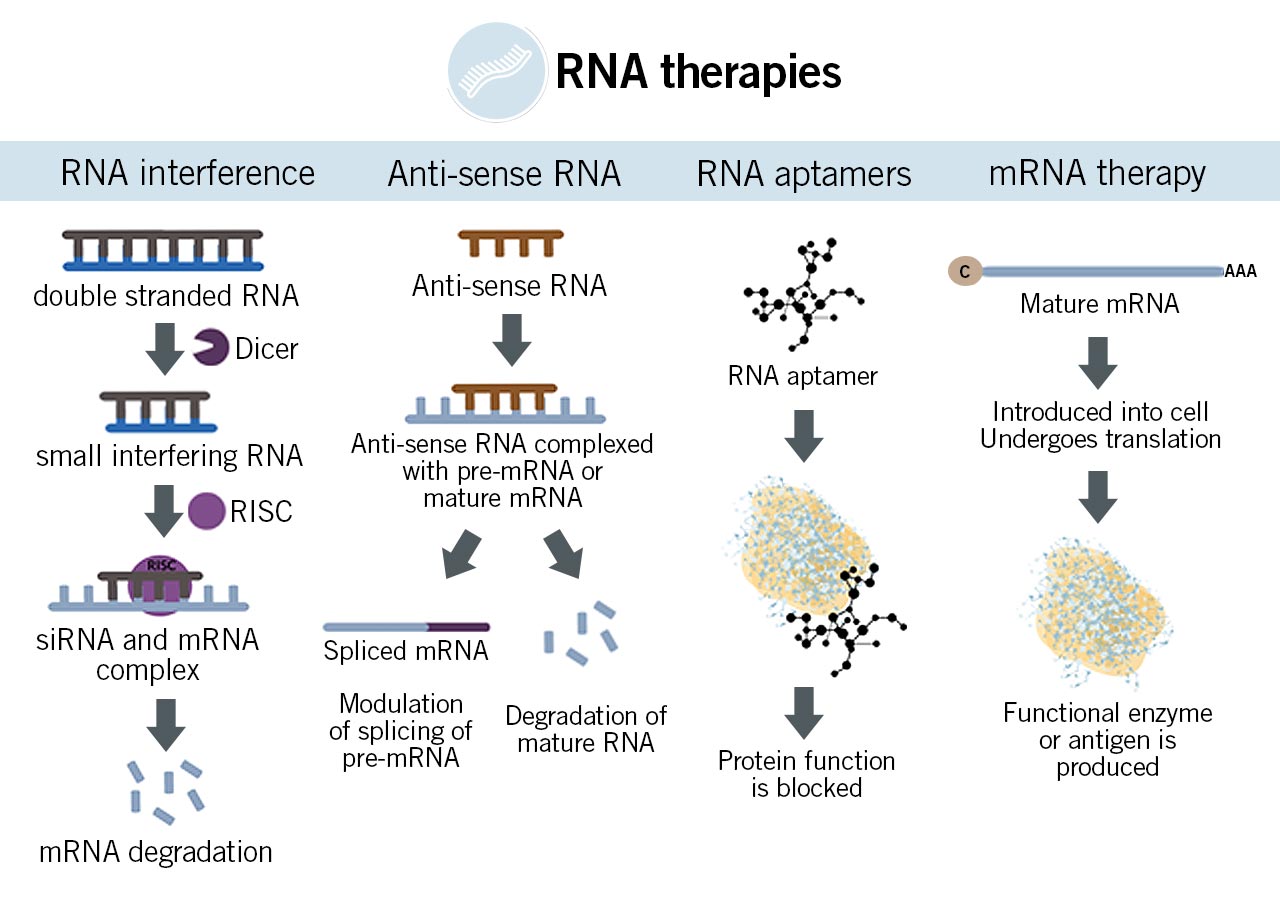
Figure: Actions of a) RNA interference, b) anti-sense RNA, c) RNA aptamers, and d) mRNA therapy
It is envisioned that RNA therapies will be used to treat diseases for which no treatment options are currently available. RNA has certain characteristics that make it superior to other available therapeutic technologies. Firstly, RNA drugs enable the targeting of both the coding and non-coding RNAs. Secondly, RNA drugs are easier and faster to design. Thirdly, and most importantly, RNA therapies are transient and don’t lead to permanent changes of the genome, and RNA drugs can produce a long-term effect comparable to conventional drugs (Kim, 2020).
For example, high cholesterol levels could lead to cardiovascular diseases, and researchers have conducted clinical trials with an RNA-based drug to lower LDL cholesterol. They found that the level of LDL cholesterol remained low even six months after a single dose treatment. A relatively long-lasting effect of the RNA drug is extremely desirable for patients who cannot manage to receive frequent doses (Fitzgerald et al., 2017; Kim, 2020). As many inherited diseases share specific mutations, RNA drugs are best suited for tackling diseases with common genetic aetiology (Harries, 2019). The design of personalised RNA drugs for individual mutations, however, won’t be cost-effective in the foreseeable future of RNA.
Future of RNA as vaccines
RNA vaccines against SARS-CoV-2 are not the first vaccines to be produced. In general, mRNA is a highly robust, reliable and efficient platform for vaccine production. A lot of research work has already been done in developing vaccines against infectious diseases and cancer. Various pre-clinical and clinical trials are underway for the development of vaccines against HIV, zika, dengue, ebola and papillomavirus.
The idea of cancer RNA immunotherapeutics is strongly advocated nowadays. By challenging cancer patients with mRNA encoding for tumour antigens, an anti-tumour immune response can be generated in the body. Moreover, a London-based biotech company, MiNA has leveraged the potential of small activating RNAs (saRNA), which can upregulate the expression of their target genes. Currently, the company has a vaccine in clinical trials that involves the use of saRNA to upregulate the expression of CEBPα in patients with liver cancer. CEBPα is the product of a tumour suppressor gene that is downregulated in cancer (Rinaldi, 2020).
Apart from these already impressive applications, what further research has been done in the field of RNA and what are the implications for the future of RNA?
Future of RNA in research
Currently, research is underway to pave the way for RNA to reach clinical application. However, there are some challenges in applying RNA. First and foremost, the delivery of the RNA molecule to the target location in the body still represents a challenge. Researchers are working on nanoparticles and conjugate-based delivery of RNA molecules. Nanoparticles can be synthesised in a variety of forms. Lipid nanoparticles are usually employed for a systemic delivery as they provide the added benefit of improved endocytosis efficiency. Another approach is to mix the negatively charged RNA with positively charged polymers to produce nanoparticles (Kim, 2020).
A conjugate-based delivery strategy is utilised when RNA has to be delivered to a specific target tissue. Here, the RNA is mixed with the specific conjugate. The conjugate acts as a ligand for receptors on the surface of the target cells that mediate endocytosis of the RNA-conjugate complex. For instance, N-acetylgalactosamine is mixed with RNA molecules destined to reach hepatocytes (a cell type of the liver). Givosiran, for example, is an RNA drug that is efficiently delivered using conjugate technology, and has been approved by the FDA for the treatment of acute hepatic porphyria (abnormal heme biosynthesis) (Kim, 2020).
Another obstacle in the way of RNA applications is the cost: RNA therapies are very expensive. For instance, the UK National Institute for Health and Care Excellence quotes 0.5 to 3 million USD for a single year of the treatment for a patient with spinal muscular atrophy using Nusinersen. However, research is rapidly progressing to bring down the cost of RNA therapies. “Advances on the scale of RNA therapies do not come cheap, and we should not underestimate the value of an effective therapy to the family of a child with spinal muscular atrophy,” wrote Lorna Harries, a molecular geneticist at the University of Exeter, UK (Harries, 2019).
Right now, RNA therapy is complex and expensive, but so were all other technologies, including next-generation sequencing. There are fantastic treasures yet to be found in the field of RNA. The future of RNA seems bright.
Eurofins Genomics provides a wide range of RNA and RNA-related services. Speak to our genomics experts.
The history of mRNA applications article – Learn how mRNA was found and what the early applications were long before COVID-19! Learn about mRNA vaccines, regenerative medicine and stem cells.

By Tamseel Fatima and Dr Andreas Ebertz
Did you like this article on the future of mRNA? Then subscribe to our Newsletter and we will keep you informed about our next blog posts. Subscribe to the Eurofins Genomics Newsletter.






One Comment