Faecal transplants for the treatment of an antibiotics-associated disease
A dysbiosis of the microbiome in the gastro-intestinal (GI) tract, or short gut microbiome, has been associated with several diseases and health conditions such as irritable bowel syndrome, inflammatory bowel disease and colorectal cancer. In order to mitigate or improve these GI-related conditions, faecal transplants have been tested in clinical trials and positive impacts were reported. For example, in hospitalised patients that were treated with antibiotics and develop GI problems caused by the bacterium Clostridium difficile, the experimental approach of faecal transplants from a healthy donor has helped and even saved these patients. Prior to the treatment, they suffered from uncontrollable diarrhoea as a consequence of the C. difficile infection. Significantly, a single faecal transplant from a healthy donor resulted in no recurrence of C. difficile infection in the majority of the patients (van Nood et al., 2013; Quraishi et al., 2017; Nishida et al., 2018; Rodiño-Janeiro et al., 2018; Zou et al., 2018; Nicholson et al., 2019).
In a clinical trial in June 2019, two patients that were infected by C. difficile were given faecal transplants from healthy donors to restore their gut microbiota and stop the diarrhoea. However, they contracted severe infections with multi-drug-resistant E. coli through the faecal transplants… and one of the patients died because of it. It must be emphasised, however, that both patients were immunocompromised when they received the faecal transplant. Nevertheless, the donated faecal samples were not properly screened for multi-drug-resistant bacteria beforehand, which could have prevented the death of the patient (Grady, 2019).
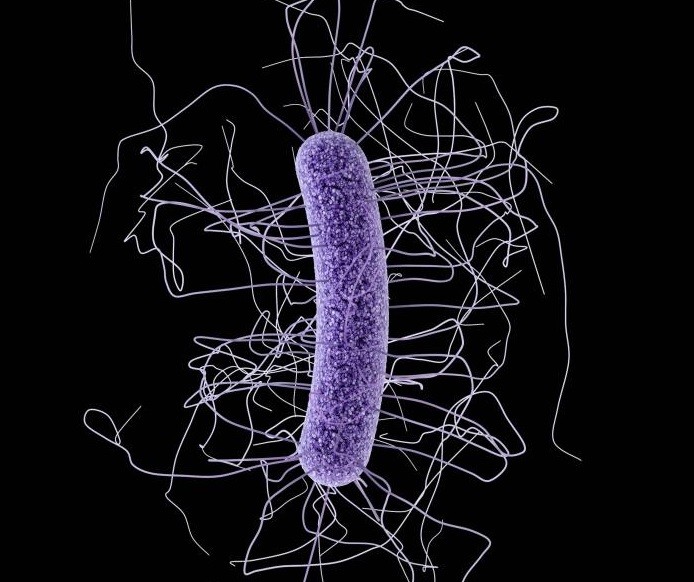
Image: Clostridium difficile
How can faecal samples be screened for multi-drug-resistant bacteria?
Faecal samples can be screened for multi-drug-resistant bacteria with the Eurofins Genomics INVIEW Metagenome service. This service is based on sequencing of the entire genomic information of all organisms present in a complex sample. Our subsequent bioinformatics analysis of the sequencing results for genes encoding for antibiotic resistances can contribute to the identification of potentially harmful microbes.
Faecal transplants for the treatment of other conditions
Beneficial effects of faecal transplants go beyond diseases of the gastro-intestinal tract. For example, a trial found that children with autism spectrum disorder experienced a long-term benefit after treatment that included the transplant of faecal microbiota from a healthy donor. Even two years after the treatment, the autism-related symptoms, as well as the children’s gut health, had improved. Overall, the treatment increased microbial diversity and the abundance of beneficial bacteria such as Bifidobacteria in the GI tract of the children (Kang et al., 2019).
What is the decisive component of faecal transplants?
It seems obvious that the microorganisms are the decisive components. Interestingly, sterile filtered faeces seem to have a similar effect as whole faecal material transplants, at least on C. difficile infections. It has been speculated that bacteriophages and postbiotics are the components that make an impact. Bacteriophages are highly specific regulators of bacteria that could affect community dynamics and contribute to a balanced intestinal microenvironment. Postbiotics are bacterial components such as bacterial debris, peptides and DNA, and metabolites that have been shown to mediate immunomodulatory effects (Tsilingiri and Rescigno, 2013; Ott et al., 2017).
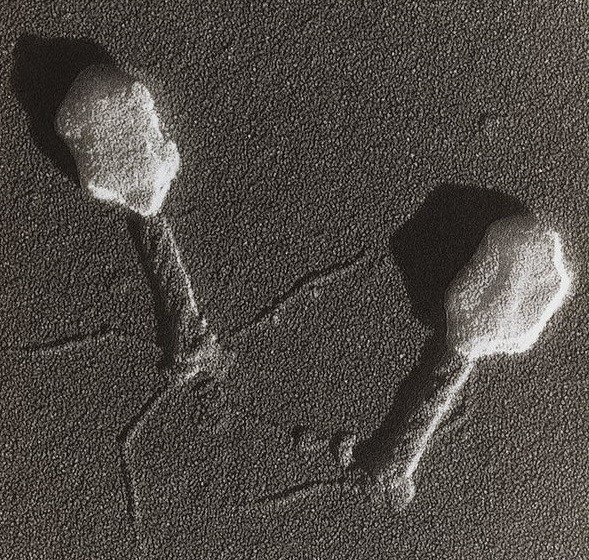
Image: Bacteriophage
Overall, faecal material and filtered faeces transplants have been shown to be beneficial for treatment of several diseases but precautions need to be taken to ensure that potential multi-drug-resistant bacteria are not transferred. In the future, specific microbiota-based formulations that contain the microorganisms and compounds that mitigate specific disease symptoms probably would be a safer and preferable alternative. Regarding the progress in microbiome research, new insights will likely be gained very quickly and could be translated into diagnostic tools directly for consumers.
How does the microbiome influence human health? What are the effects on metabolism, immune system and inflammation?
By Dr Andreas Ebertz
Did you like this article? Then subscribe to our Newsletter and we will keep you informed about our next blog posts. Subscribe to the Eurofins Genomics Newsletter here.




3 Comments