Cancer ecology: A different picture of tumour development
Tumours are the second leading cause of death in western societies, just after cardiovascular diseases. For decades, scientists and physicians have given their very best to find effective treatments. Every now and then, new promising therapies and strategies arise, but still, cancer remains the synonym for a long and painful fight for life that is rarely won.
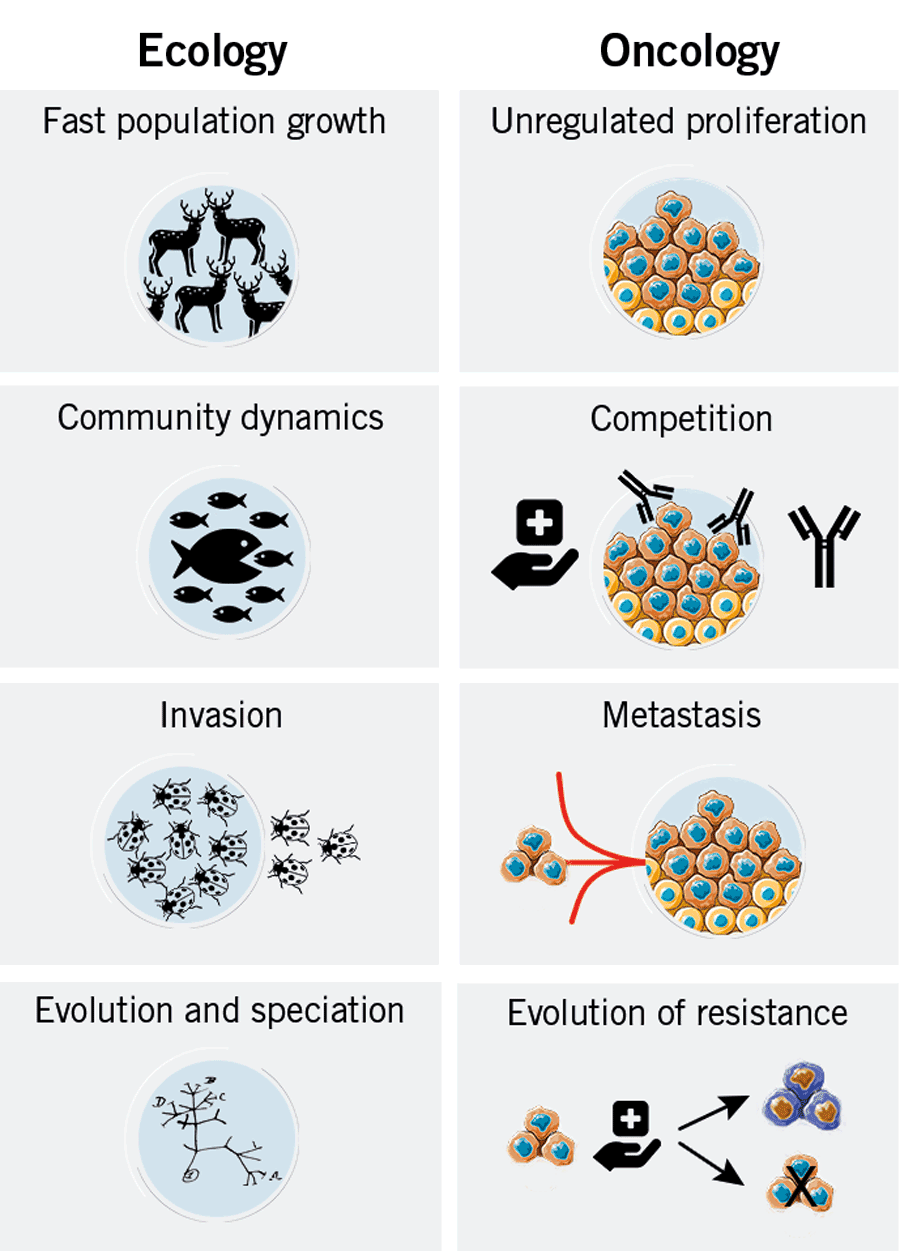
Therefore, some scientists suggested a change to our understanding of cancer biology to find more suitable approaches that improve life expectancy of patients. Cancer can be seen as a population of cells that are highly depend on their surroundings. Ecological principles are being recognised as a suitable means to describe different stages of cancer progression. This is called cancer ecology.
Evolution of cancer: In the light of cancer ecology
In 1976, Peter Nowell proposed an intriguing idea that tumour cells are subjected to clonal evolution and that a tumour is, in fact, a population of evolving tumour cells. Since then, this idea has been widely accepted and evolutionary biology entered the field of oncology.

Image: Professor Peter C. Nowell, MD (1928-2016). Reproduced with permission from the Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania (Philadelphia).
Based on our knowledge of evolutionary theory, we know that evolution happens in tune with the environment, and tumour cells are also under constant selection pressure from their environment. The environment consists of biotic and abiotic factors. Biotic factors, for example, are the increased presence of macrophages, blood vessels and so on. Abiotic factors are the amount of oxygen, nutrients as well as therapeutic compounds, such as cytotoxic drugs for example (Bain, 2017; Somarelli, 2021). All those factors are present in a complex and dynamic interplay in cancer ecology.
Cancer ecology: Tumours in their natural environment
Tumours are radically different from normal tissues. Besides a changed architecture of the normal tissue surrounding the tumour, the tumour itself also shows an altered architecture (figure 1). Tumours attract and employ normal cells that provide them with the necessities for tumour progression. By employing different host cells, tumours arrange their environment, a phenomenon called niche construction. A famous example of niche construction from the animal kingdom is dam building beavers. By building such elaborate structures, beavers change their environment and modulate the selective pressure of the environment that acts on them. Similarly, the selective pressure on tumour cells changes with niche construction and generally favours tumour progression. For example, tumour-associated macrophages (TAM) produce a plethora of compounds, such as vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF), that promote tumour growth and invasiveness. Along with tumour cells, TAMs produce proteases that help to digest proteinaceous stroma (supporting connective tissue of an organ) and allow invasive tumour cells to migrate to distant environments and eventually metastasise.
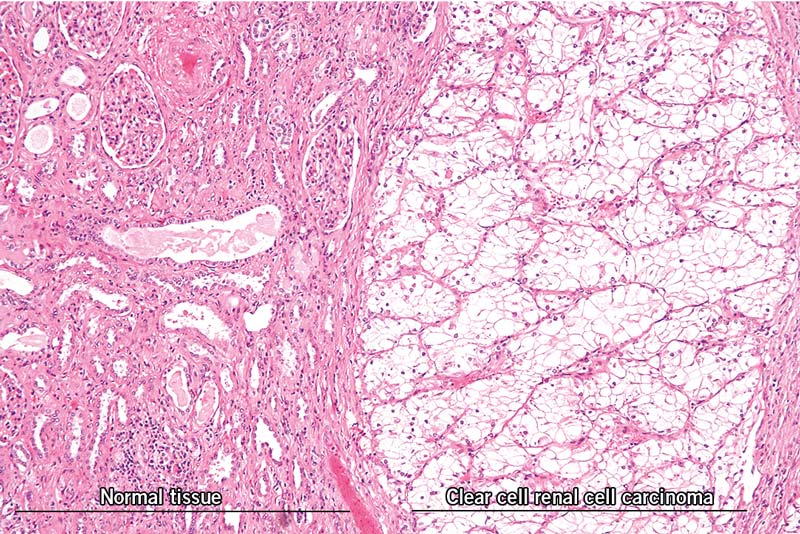
Figure 1: Image of a clear cell renal cell carcinoma
One of the most recognised alterations of a microenvironment that tumours induce by secreting soluble factors, such as VEGF, is angiogenesis (figure 2). Angiogenesis is a process of ingrowth of new blood vessels into the tumour tissue, which provide tumour cells with enough oxygen and nutrients to thrive. In general, a tumour tissue is very heterogeneous, with multiple environments that are characterised by different biotic and abiotic conditions. For example, blood vessels are not evenly spread inside a tumour tissue so not all cancer cells get access to the same amount of oxygen; some areas of a tumour might even become hypoxic. A hypoxic microenvironment is thought to select the most invasive and aggressive tumour cells. The ecological explanation for this is simple: when essential necessities are scarce and competition among cancer cells is strong, a cancer cell population can migrate to find a nourishing and less competitive environment.
Furthermore, when stimulated by tumour-derived factors, collagen-producing fibroblasts develop into myofibroblasts that give the tumour mass a certain stiffness that is usually recognised as a lump (Somarelli, 2021; Paraic et al., 2006).
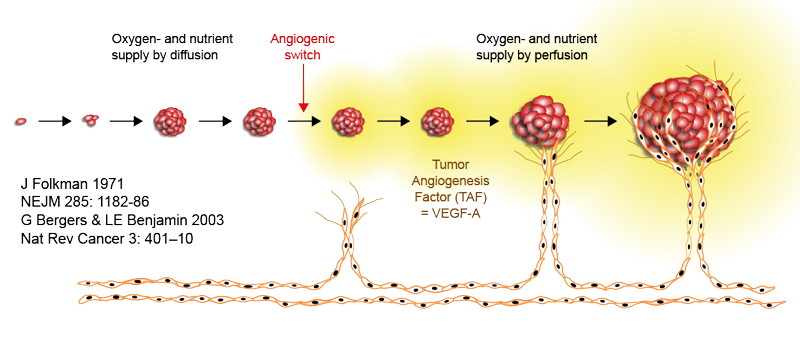
Figure 2: Schematic representation of cancer induced angiogenesis.
Cancer ecology: Applied lessons from traditional ecology
There are some striking similarities between cancer ecology and conventional ecology. Different areas of ecology can be applied to describe cancer. For example, the growth of a tumour cell population can be described in the same manner as the population growth of a species.
Generally, when a population is not crowded it grows exponentially. As the population gets crowded, competition among individuals becomes stronger and stronger, thus the population growth stagnates and reaches a plateau. In cancer, a stagnation does not necessarily mean that the tumour stops growing. It just slows down due to an ever changing selection pressure and extinction of non-adapted cancer cell lines.
Cancers are extremely crowded tissues and the competition of cancer cells among themselves and with normal cells slows down rapid cancer growth. Similar to species population, the tumour environment (organ or normal tissue) has a certain carrying capacity that is defined as the maximal population size before it starts to destroy the environment. There are plenty of examples of this in the natural world. Before the Europeans colonised North America, the populations of North American deer were kept in balance by wolves. The settlers, however, saw wolves as danger to their cattle and communities and began to exterminate wolves at a rapid pace. Needless to say, the population of North American deer skyrocketed and, as they need large amounts of vegetation to sustain themselves, they began to deplete their food sources and eventually starved.
When the tumour environment exceeds carrying capacity, the tumour-bearing organ begins to suffer more and more damage (figure 3). Organ damage can happen in different ways: by simply pressing on the surrounding tissue, by blocking important ducts, restricting the inflow of nutrients and oxygen, or by secreting different factors that negatively affect the homeostasis of an organ (Reynolds et al., 2020).
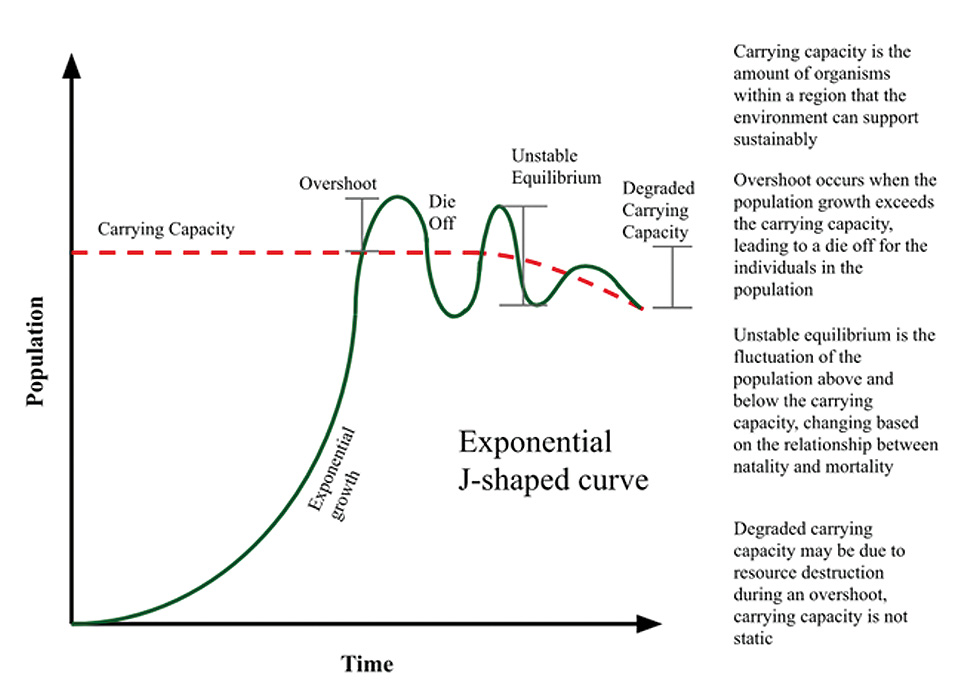
Figure 3: Graphical representation of environment destruction caused by overpopulation.
Cancer ecology: Metastasis from an ecological point of view
Metastasis is an ecologically unique process, and it has some intriguing similarities with the migration of invasive species. Successful invaders have some traits in common, such as fast growth, fast maturation and reproduction, long-distance dispersal capabilities, resistance to predation and adaptability. All of this is also typical for cancer cells.
However, an invader with all those traits still cannot invade radically different or non-permissive environments. This also applies to cancer cells. Therefore, different cancer types have preferred organs for metastasis (figure 4). This has been known for decades as seed and soil theory. The seed represents the invading cancer cells and the soil represents the suitable organ/environment in which the seed can “germinate”. Also, some organs seem to be much better soil for metastasis, such as the brain and bones. An explanation for this could be that those organs have low cell density, providing cancer cells with lots of space to proliferate, and are well supplied with blood vessels that provide nutrients and oxygen.
And yet another lesson we can learn from biological invasion is that devastated environments are the most vulnerable to invasions! The reason is that the native species lose their usual niches, and thus predation, among other biotic factors, is reduced. Similarly, metastasis in a diseased organ is much more likely to happen than in a healthy organ. Finally, some diseases result in immunodeficiency and reduced anti-tumour immunity, and therefore circulating and metastasising cells are less exposed to “predation” by the immune system.
These are just some examples of the similarity between traditional ecology and cancer ecology. But how can cancer ecology knowledge be utilised to modify traditional cancer therapy (Somarelli, 2021; Reynolds et al., 2020)?
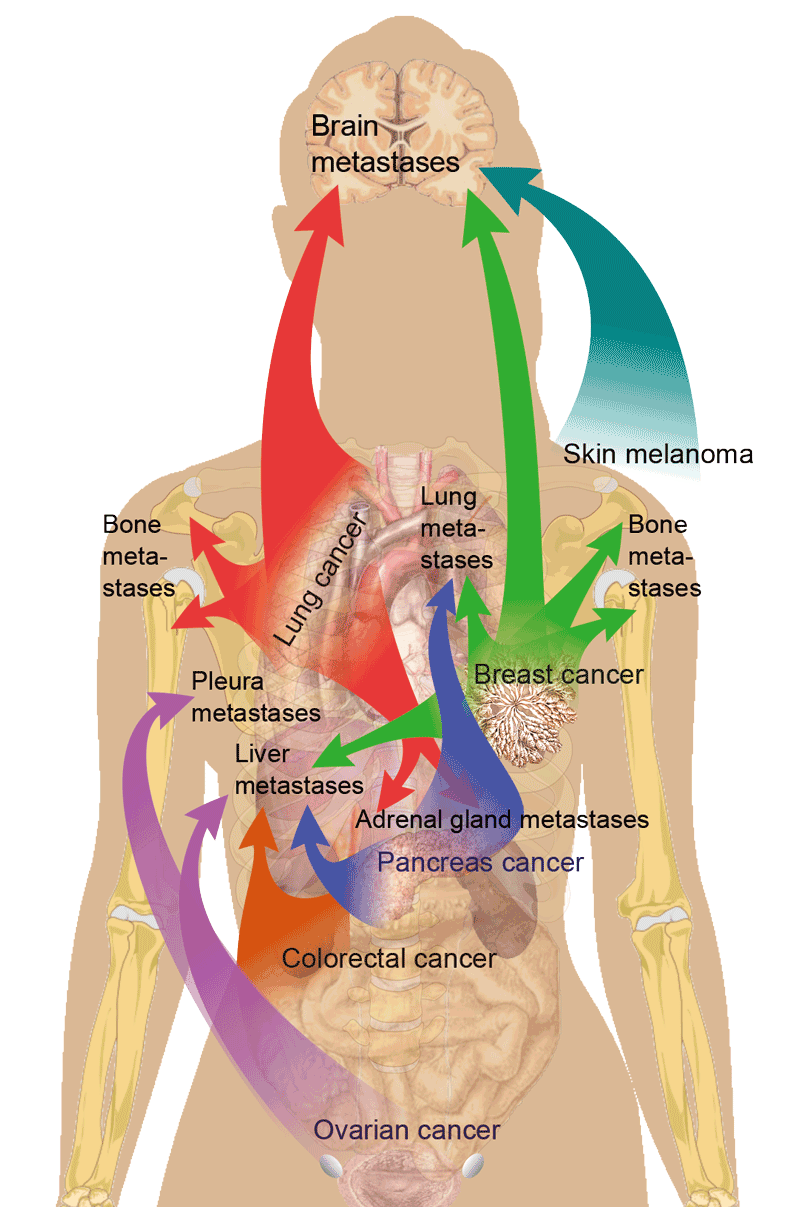
Figure 4: The most usual places of metastasis for different types of cancer.
Cancer ecology: Impact on cancer therapies
There are a lot of therapies that are very good at killing cancer cells, but often the problem is that these therapies usually have a substantial negative impact on normal cells as well. These negative side effects are heavily correlated with the dosage of the therapeutic agents. Up until recently, chemotherapies and radiotherapies were given at the maximal dosage that a patient can tolerate. This approach is extremely hard for patients and usually weakens the patient’s organisms and induces immunodeficiency, which makes them vulnerable to other diseases.
Another problem of maximum dosages is that this changes the selection pressure on the cancer cells in a way that it favours the survival of therapy-resistant cancer cells. Non-resistant cells die off, and pretty soon the cancer becomes resistant to the therapy. The knowledge of cancer ecology and recognition of competition between cancer cells has already had a big impact on the dosing of therapeutic agents. By lowering the dosage, side effects become more tolerable and the non-resistant cells do not get fully eradicated. Thus, the non-resistant cells continue to compete with the drug-resistant cells, keeping them at bay. That way, drug-resistant cancer cells do not become the only type of cells in the tumour, and standard therapies can still be used to control the cancer.
There are several new approaches of dosing the therapeutic agents. For example, metronomic therapies are characterised by applying cytotoxic agents at much lower, but more frequent doses. Conventional therapies are given at maximum tolerated dosages in about 2 or 3 week intervals. In metronomic therapies, on the other hand, the therapeutic agents are given every day, or every other day, at much lower dosages. This way, the blood concentration of the drug remains relatively constant, making it more bearable for the patient.
Another, more recent approach is adaptive therapy, in which the dosages are applied at the minimum necessary amount needed to manage the symptoms. This kind of therapy also prevents angiogenesis and induces a stronger immune response to the cancer.
Adaptive and metronomic therapy both have been shown to provide better life quality compared to conventional therapies. Regarding life expectancy, these approaches show significant increases in some cases, although it depended on the type and stage of the cancer (Maiti, 2014; Simsek et al., 2019; Parikh et al., 2016; Kim et al., 2020).
The current paradigm in cancer treatment is to change the treatment for cure strategy to treatment for stability, where the population of non-resistant cells is maintained and thus cancer stays manageable. After all, cancer is a chronic disease and patients are only rarely fully cured, especially when diagnosed at later stages. The goal is, by development of better therapeutic strategies, to slow down cancer progression and to manage symptoms so that patients can continue with their day-to-day lives (Reynolds et al., 2020; Somarelli, 2021).
Conclusion on cancer ecology
Cancer is one of the biggest problems of modern medicine, and big problems often require a paradigm change over time. In the past, cancer was considered to be just a mass of rapidly proliferating cells, but as time went by we learned that a tumour is much more complex and an evolving cluster of cells that is dependent on its environment. This lead to the modification of existing therapies that are not primarily designed to eradicate cancer, but to keep the disease stable with respect to ecological interactions that keep the drug-resistant cells from proliferating. It will be interesting to see what the future holds, but it is certain that we have a lot more to learn from the natural world. The field of cancer ecology will eventually provide new insights in the fight against cancer.
Find out about the Eurofins Genomics’ oncology solutions to answer any genomic or transcriptomic question.
By Matko Tudor and Dr Andreas Ebertz
Did you like this article on cancer ecology? Then subscribe to our Newsletter and we will keep you informed about our next blog posts. Subscribe to the Eurofins Genomics Newsletter.






One Comment