Key takeaways:
- Glucose and fructose metabolisms are different. Glucose intake leads to increased energy levels in the cell, whereas fructose leads to reduced energy levels.
- Fructose intake can contribute to increased fat storage, insulin resistance and high blood pressure.
- It has been shown that many cancer types prefer fructose as fuel.
- Sweet beverages contain high amounts of sugar and are consumed quickly. Avoiding soft drinks and fruit juices is an excellent way to reduce sugar intake.
Sugar metabolism in the cell – This is where the scientific fun starts
Even though glucose and fructose structures are fairly similar, their metabolisms are fundamentally different.
Glucose intake results in an increase of energy in the cell that metabolises it.
In contrast, fructose intake lowers the energy in the cell. In fact, it is the only nutrient that does this!
From a superficial point of view that does not make sense. Both are sugar molecules and sugar provides energy for ATP generation.
That is true for glucose, as during its metabolisation (glycolysis), ATP is eventually produced. However, in order to make ATP, some energy in the form of ATP has to be spent first. These are steps 1 to 3 during glycolysis (image 1) and are called the investment phase.
To prevent a significant ATP depletion in the cell when a large amount of glucose is metabolised during steps 1 to 3, a cellular mechanism stops the metabolisation of glucose at an early point – step 3 (image 1). The key enzyme of this stop mechanism is phosphofructokinase (PFK). It is downregulated and turned off if cellular ATP levels decrease too much. The glucose that is already in the glycolysis pathway (after step 3 in image 1) is used to generate ATP, and, therefore, the total ATP levels within the cell increase.
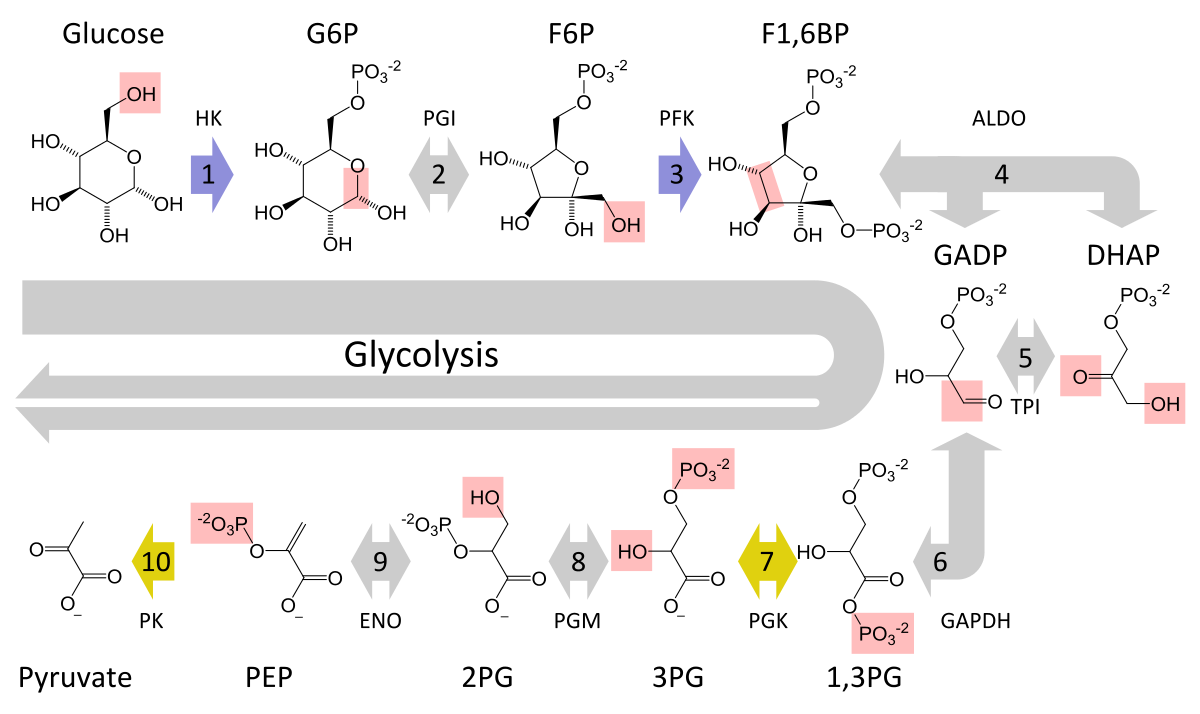
Image 1: Depiction of glycolysis that converts glucose via intermediate metabolites to pyruvate. Steps 6 to 10 occur twice per glucose molecule.
Fructose metabolism, called fructolysis, is deeply different. Here, the key enzyme fructokinase consumes ATP in an unregulated way. ATP levels in the cell can plummet up to 50% during fructose metabolisation. It is like the bitcoin crash from April to July 2021. As so many investors experienced at the time, it is like a mayday signal throughout the body. The system switches to a state of preserving resources. Metabolism and energy expenditure are reduced and the calories from the eaten food are channelled into glycogen and fat storage instead of ATP production. The body is doubling down, triggering hunger and thirst to eat more in order to restore ATP levels.
In nature, animals eat fructose in autumn to store fat and prepare for the cold season. It is a survival mechanism and can be best observed in migrating birds. Fructose consumption induces insulin resistance, hunger and slowed metabolism, all contributing to increased fat storage. During migration, the fat is used for energy production. This fructose mechanism is independent from the uricase mutation in humans and great apes (Johnson et al., 2020).
In addition, and in conjunction with the falling ATP levels in the cell, the intracellular phosphate levels also fall during fructolysis, and phosphate is central to the recycling of ATP (image 2).
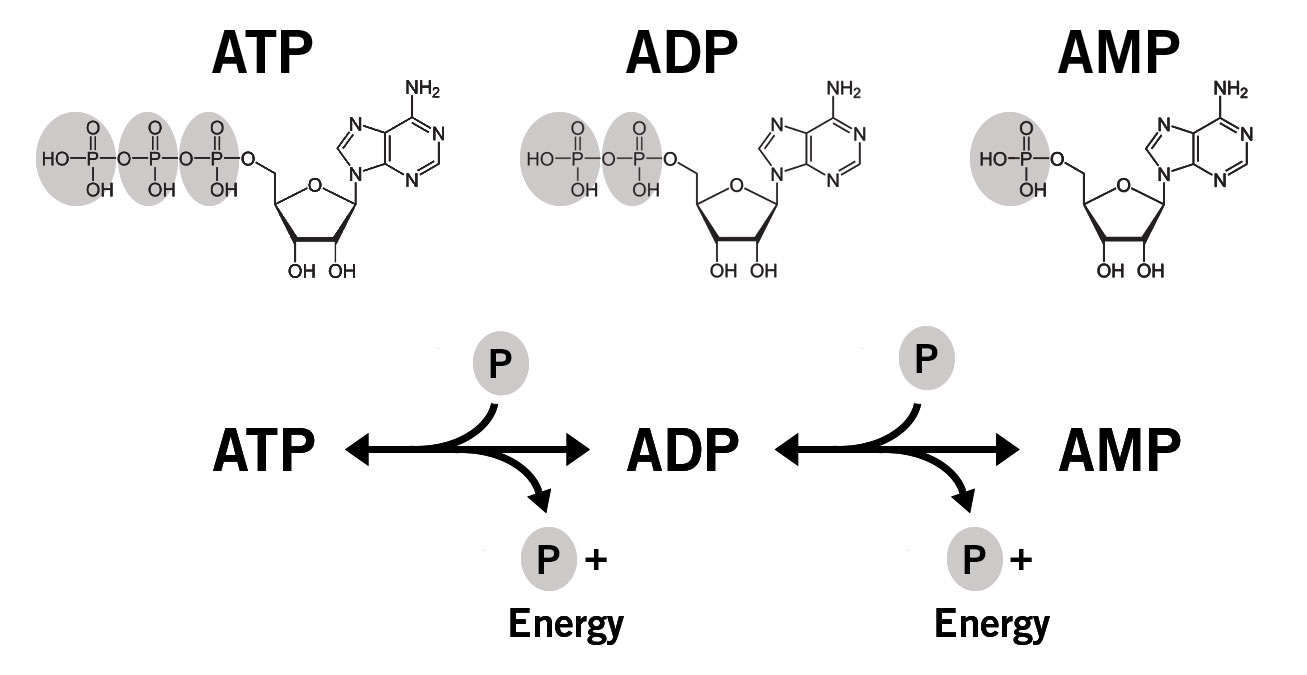
Image 2: Simplified depiction of ATP recycling in the cell. P = phosphate group.
Note:
Adenosine monophosphate (AMP) can be produced by:
- Cleavage of a phosphate bond in ADP (ADP à AMP + Pi)
- Hydrolysis of ATP to form AMP and pyrophosphate (ATP à AMP + PPi)
- Transfer of one phosphate from one ADP molecule to another ADP to form ATP and AMP
Hydrolysis of ATP or ADP to form AMP releases energy that the cell can use.
ADP is regenerated by reaction of ATP and AMP to form 2 ADP molecules, which can then regenerate ATP in the mitochondria or during glycolysis.
The low intracellular phosphate levels activate the enzyme AMP deaminase that converts AMP into uric acid. Instead of a regeneration of ADP from AMP via a kinase, AMP is degraded to uric acid. The free intracellular phosphate is taken up by fructose to produce fructose-1-phosphate (image 3). Significantly, this pathway of AMP degeneration also seems to be involved in the illnesses and ailments prevalent in the developed world: insulin resistance, obesity, fatty liver disease and elevated blood pressure (Lanaspa et al., 2012).
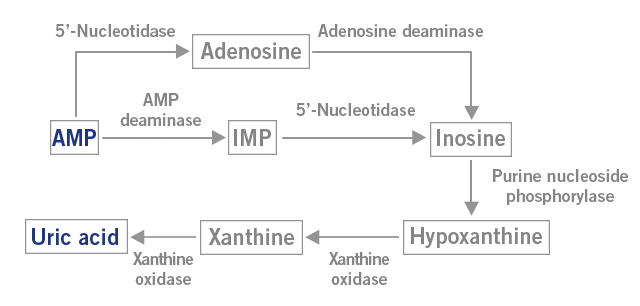
Image 3: Depiction of the AMP metabolism to uric acid.
Sugar and metabolic syndrome – The global epidemic
A person suffers from metabolic syndrome, if three of the following five symptoms are diagnosed:
- Abdominal obesity (high waist circumference)
- High glucose levels in the blood (insulin resistance)
- High blood pressure
- High serum triglycerides
- Low HDL cholesterol
The most severe diseases associated with metabolic syndrome are cardiovascular disease and type II diabetes.
The link between fructose and increased fat storage, insulin resistance and high blood pressure has been described earlier in the text. But the effects of fructose and uric acid are even broader.
What comes next, might need to be read twice to be fully digested.
Fructose-induced metabolic syndrome does not need an increased calorie intake!
In rats that are prone to developing metabolic syndrome as they age, even a caloric restriction in combination with a high sucrose diet resulted in high triglycerides, fatty liver and type II diabetes; all metabolic syndrome-associated symptoms. The control group that received the same amount of calories (isocaloric diet) without sucrose did not develop these symptoms.
In the sucrose-fed rats, the researchers found low grade inflammation in the insulin-producing pancreatic islets that simultaneously showed an upregulation of membrane proteins responsible for uric acid uptake (urate transporter URAT1). This suggests that sucrose (50% fructose & 50% glucose) causes insulin resistance and simultaneously affects pancreatic islets directly by increasing uric acid concentration. These findings could mean that even a mild caloric restriction for weight loss purposes could lead to fatty liver and insulin resistance when the sugar intake is not restricted.
Similar to the rat study, an epidemiological study in humans across 175 countries suggested a link between diabetes and the availability of sugar independent of total calorie consumption (Roncal-Jimenez et al., 2011; Ghasemi, 2021).
Still not convinced to reduce your sugar intake? Let’s have a look at the effects of fructose on mitochondria, the key elements for energy production in the body.
Sugar and mitochondria – How to impair the principal site for nutrient metabolism and energy production
The leading cause of death in the developed world is heart disease; and the key to a healthy heart are functional mitochondria. Around 40 % of the cytoplasm space in heart muscle cells is occupied by mitochondria! Apart from disease, mitochondria play a vital role in athletic and everyday performance. After all, a large majority of the energy used daily is provided by mitochondria (Brown et al., 2017; Poznyak et al., 2020; Lin et al., 2021; Bscb.org., 2022).
Fructose and its metabolite uric acid have been found to profoundly impair mitochondrial function. In muscle and liver cells for instance, fructose and uric acid lead to mitochondrial oxidative stress that can damage mitochondrial DNA and even lead to decreased mitochondrial DNA content. The mitochondrial dysfunctions include the reduction of fatty acid uptake and degradation, reduced citric acid cycle and activity of the respiratory complexes – the three main process of energy production in the mitochondria (Lanaspa et al., 2012 a; Lanaspa et al., 2012 b; Jaiswal et al., 2015; Johnson et al., 2019).
Note: Fatty acids are metabolised in a process called beta-oxidation to generate coenzymes for ATP production and acetyl-CoA, which is the starting compound for the citric acid cycle (image 4).
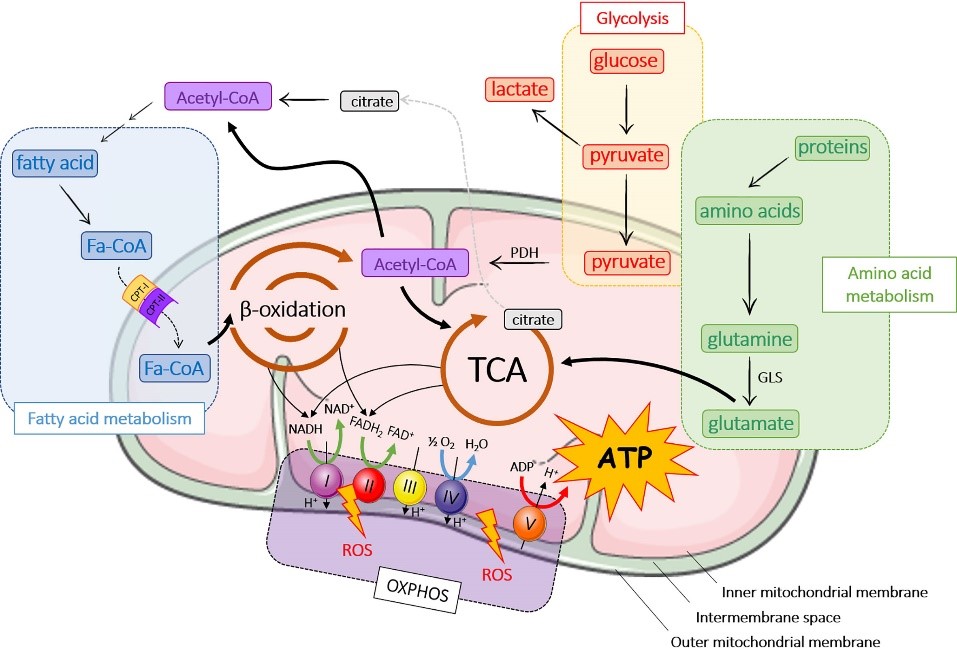
Image 4: Depiction of the interplay of the metabolic processes beta-oxidation, citric acid cycle and electron transport chain.
Overall, fructose consumption leads to mitochondrial dysfunction and impaired energy metabolism.
This leads to another alarming issue. In addition to the mitochondrial dysfunction, fructose seems to stimulate glycolysis for energy production. Glycolysis, the process of glucose metabolisation that occurs outside of the mitochondria in the cytosol, is sometimes mentioned in connection to cancer.
Fructose and cancer – The Warburg effect
It is widely known that the metabolism of cancer cells is shifted towards glycolysis. Here, pyruvate, the end product of glycolysis, is mainly converted into lactate instead of being utilised in the mitochondria for further energy production. This is called the Warburg effect (image 5).
Significantly, fructose seems to also shift the cell’s metabolism more towards glycolysis.
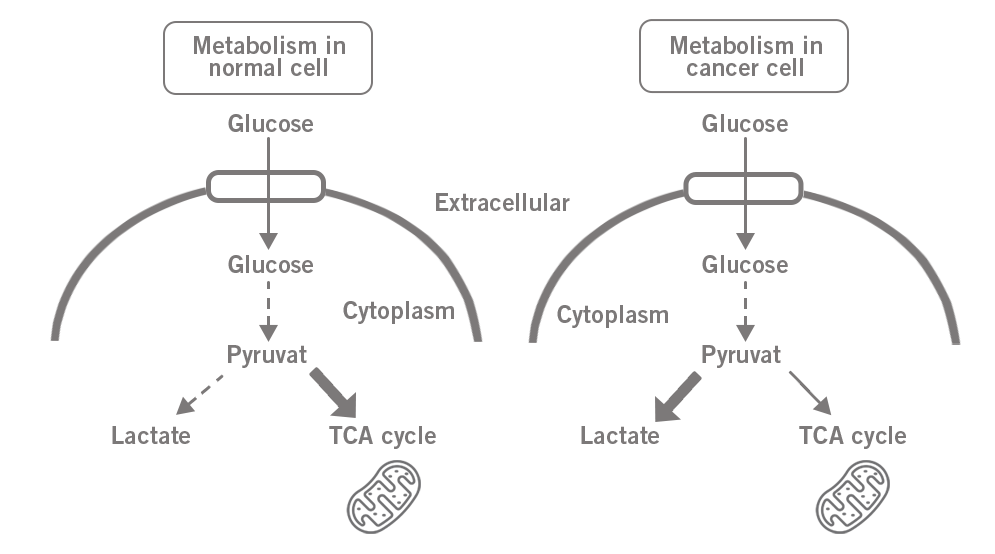
Image 5: Difference of glucose metabolism in a) normal cell and b) in cancer cell (Warburg effect).
Many cancers such as colon, kidney, liver, breast and brain cancer seem to prefer fructose as fuel. This has been substantiated by the observation of upregulated fructose transporter (GLUT5) on cancer cell surfaces. Once fructose is transported into the cell, it induces signalling pathways associated with inflammation and oncogenicity.
Furthermore, uric acid, the by-product of fructose degradation, contributes to the inhibition of the citric acid cycle in the mitochondria. Other metabolic pathways of fructose are maintained and could promote cancer cell proliferation (Nakagawa et al., 2020; Muriel et al., 2021)
By now, it should be obvious that sugar consumption is detrimental and harmful for humans in the modern world.
The fructose metabolism evolved as a survival mechanism, but how do we handle it in the 21st century?
Sugar it in the 21st century – How to avoid the harmful effects of sugar
The simple answer to the question is: Don’t eat sugar!
As long as you don’t have a mutation in the fructokinase gene that renders it non-functional, stay away from sugar. By the way, this mutation exists in humans, however, it is rare. People with this mutation seem to be immune to the effects of fructose consumption.
Note: Before blood sugar levels were measured with glucose monitors, physicians had to test the urine of their patients for sweetness by tasting. Sweet urine indicated diabetes and at that time, this was a death sentence as insulin was not available yet. However, people with the fructokinase mutation had sweet urine but no diabetes. That is how this rare condition was discovered.
Is it really that simple? Can a single molecule be, on one hand, responsible for the survival of the human species and, on the other hand, a major factor of metabolic health issues nowadays? Does the exclusion of sugar from our diet solve these problems?
Yes, it could be!
It is very hard to avoid fructose and it requires awareness. Sugar, and therefore fructose, is in many packaged foods.
An excellent measure to reduce sugar intake is to avoid drinking it. Sweet beverages such as soft drinks and fruit juices contain high amounts of sugar. By drinking them, the organism is “flooded” with a lot of sugar in a short time. This will result in a massive spike in blood glucose and fructose concentration, triggering and driving the harmful mechanisms described in this article.
Alternatively, you could just have a little sip of a sugary beverage every 10 minutes to avoid the sugar spike. But I haven’t met anyone who does this!

Therefore, you could consider this as the hard line to never cross. No soft drinks with sugar and no fruit juices! From there, you can progressively reduce your sugar consumption. The occasional sweet is surely acceptable, as long as it is not a daily habit. However, some of us are not people of moderation and cannot just eat one piece of chocolate. It may be necessary to avoid sweets completely, otherwise the “floodgates will open”. Sad but true. “Know thyself”, so you can act accordingly.
Good luck and we would like to hear about your experience of reducing your sugar consumption. Leave a comment!
Gene expression analysis is an excellent approach to investigate the transcriptional differences between two or more treatments in your research organism of choice, or the transcriptional reaction of a loss-of-function or gain-of-function mutant for instance.
We provide a wide range of predesigned or customised expression arrays for all organisms: Expression arrays.
Or use NGS-based RNA sequencing: INVIEW Transcriptome.
Disclaimer: This text is for general information purposes only and does not constitute the practice of medicine or health care services including the giving of medical advice. The use of this information is at the user’s own risk and is not intended as a substitute for medical advice, diagnosis or treatment.
By Dr Andreas Ebertz
Did you like this article about sugar? Then subscribe to our Newsletter and we will keep you informed about our next blog posts. Subscribe to the Eurofins Genomics Newsletter.







One Comment