Key takeaways:
- Glucose and fructose act very differently in the body. Glucose is a molecule of energy production and fructose is a molecule of energy storage.
- During evolution, human ancestors acquired a gene mutation that made them very sensitive to fructose. This mutation was a great evolutionary advantage.
- Diseases of modern history such as gout and high blood pressure are linked to sugar consumption.
Sugar is not sugar – The devil is in the detail
The main forms of sugar in nature are glucose and fructose. These are also the forms that are mainly used by humans, and even though the structural formulas of the both molecules look very similar (image 1), their functions in the body cannot be more different!
Glucose is a molecule of energy production. It circulates in the blood and is mainly stored in the liver, kidneys and muscles.
Fructose on the other hand is a molecule of energy storage rather than production. This has major implications. Let that sink in! Eating fructose triggers physiological mechanisms that favour energy storage rather than energy production. The energy is mainly stored as glycogen and fat!
Hibernating animals are known to eat large amounts of ripe fruit before they hibernate. This allows them to store energy, specifically from fructose, in the form of fat for the months to come. Orangutans, as close relatives to humans, have also been found to eat large amounts of fruit in order to store fat.
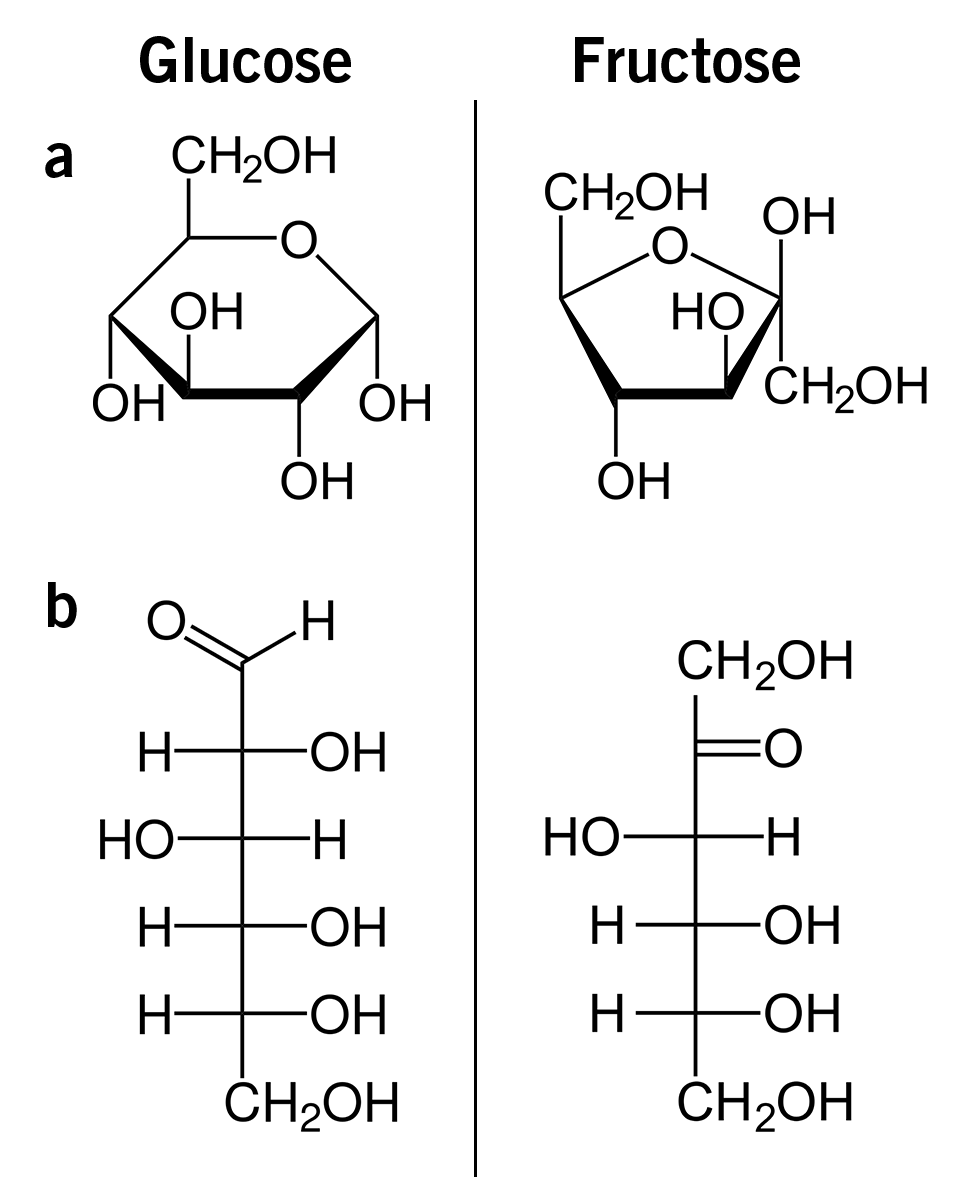
Image 1: Structural formulas of glucose and fructose as a) Haworth projection, and b) as Fischer projection.
The infamous high-fructose corn syrup
The infamous high-fructose corn syrup is a combination of glucose and fructose. When a glucose molecule is chemically linked to a fructose molecule it is called sucrose, also known as table sugar (image 2). In nature, sucrose is found in sugar beet and sugar cane. High-fructose corn syrup, in contrast, is industrially produced from corn starch.
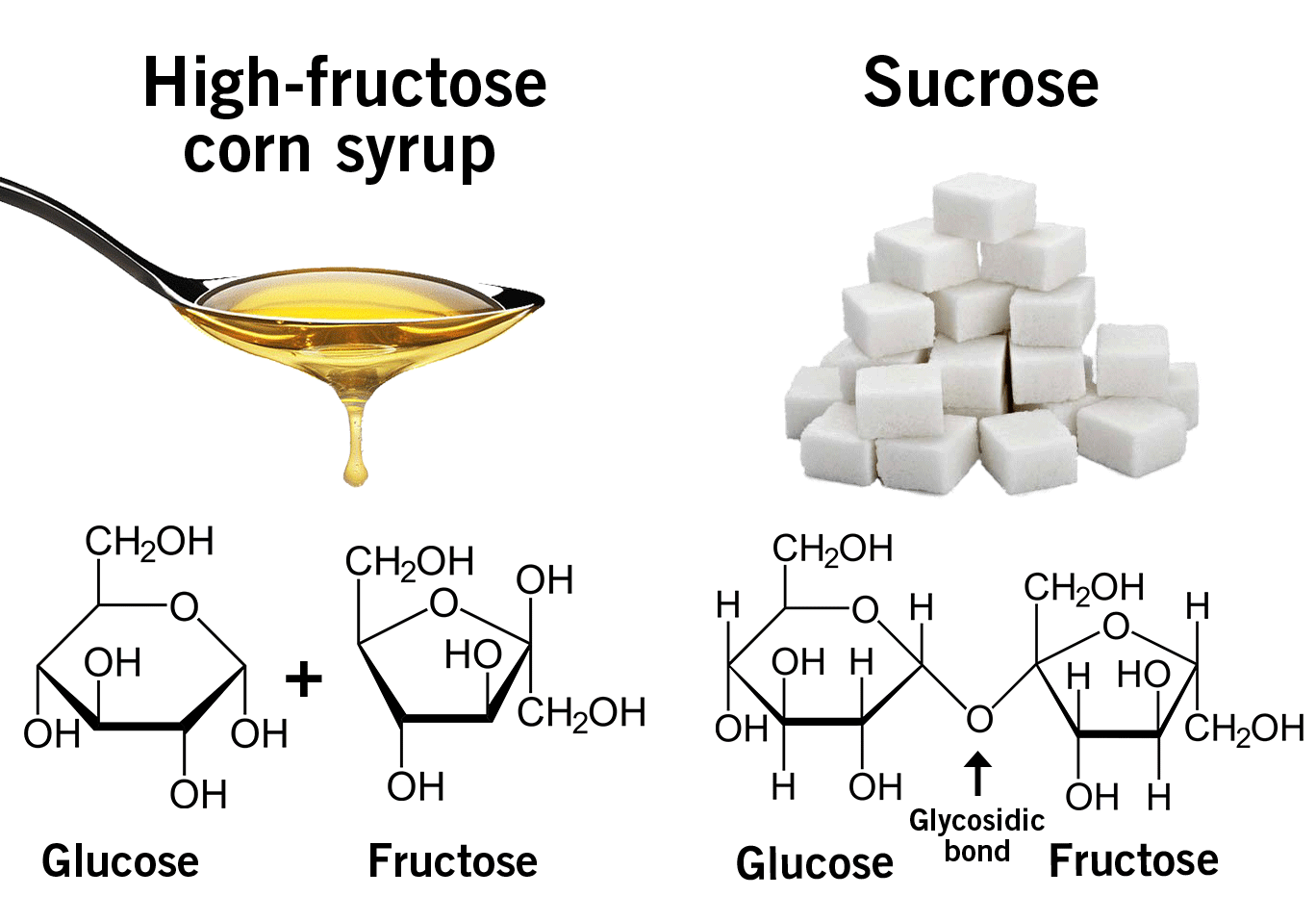
Image 2: Difference between high-fructose corn syrup and sucrose.
Sugar and evolution – The original Black Friday
Around 20 million years ago, now-extinct arboreal apes existed in West Africa who primarily ate fruit. Due to the global cooling at this time, called the big chill, land bridges emerged and connected Africa with Europe and Asia (when ice forms, sea levels fall). Many species, including these apes, migrated from Africa to Europe around 15 to 16 million years ago, a period called the Middle Miocene. At this time, the global temperature increased intermittently – a brief respite from the big chill. In the vast forests and woodlands of Europe, the emigrating apes found plenty of fruit all year around.
However, around 12 million years ago, the global cooling started to reach a point where the cold seasons were longer and more extreme in temperature. Subsequently, the apes could not find enough fruit and started to starve during the cold seasons in Europe.
Eventually, 8 million years ago at what is now known as the original Black Friday, the last individual of these apes died, resulting in the complete extinction of the species in Europe and Asia (image 3) (Andrews and Johnson, 2020; Johnson et al., 2020).
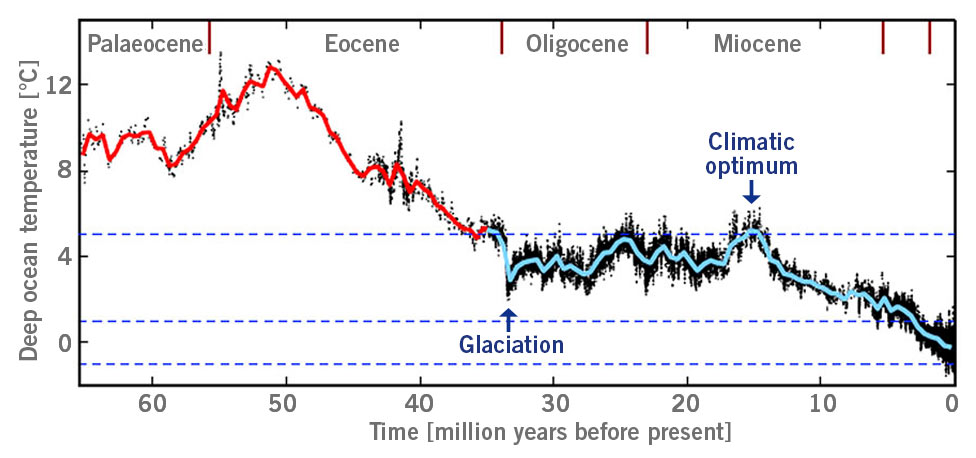
Image 3: Estimated deep ocean temperature from Palaeocene to Miocene, glaciation = large-scale glaciation of Antarctica; climatic optimum = time period with high temperatures, e.g. today’s region of Germany with annual terrestrial temperature of around 20°C or higher and winter temperature of higher than 10°C (according to Hansen et al., 2013).
In the face of this tragedy, Hermann Hesse’s declaration “A magic dwells in each beginning, protecting us, telling us how to live” rings true.

Sugar and evolution – An emerging human superpower
During this epoch and before they became extinct, evolution continued as it always does, and beneficial mutations emerged in the apes. One of the most important mutations affected the uric acid metabolism.
Note: Uric acid is mainly known in connection with gout, a form of arthritis that often starts in the big toe.
When fructose is metabolised in the liver, uric acid is formed. Here, the gene encoding for the enzyme uricase (urate oxidase) that would further degrade uric acid was mutated and silenced (inactivation). As a result, much more uric acid was produced in the liver after fructose consumption. This new ability was an evolutionary advantage, as uric acid drives fat storage from fructose. The apes with this uricase mutation could store much more fat from fruits and therefore had a better chance of surviving the long winters. This mutation could really be called a superpower necessary for survival (image 4) (Johnson and Andrews, 2011).
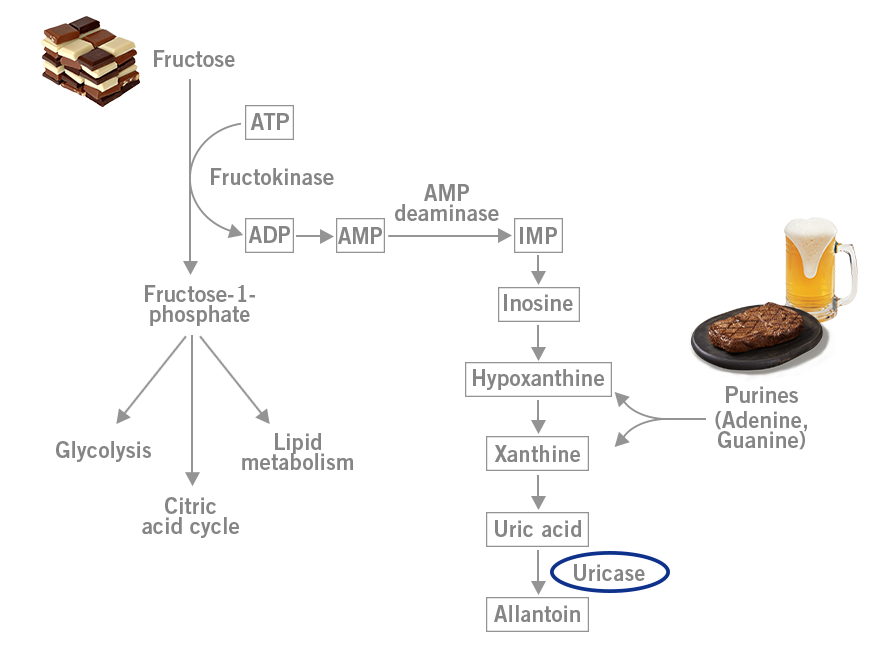
Image 4: Depiction of uric acid production during fructose metabolism in the liver.
Even if this uricase mutation was a big advantage and greatly increased the fitness of these apes, why did they become extinct and why are they not our evolutionary ancestors?
Recent studies suggest that some of the apes that had the uricase mutation migrated back to Africa and became the common ancestor of humans and the great apes that now live in Africa (e.g. chimpanzees) and Asia (e.g. orangutans). This is called the Back-to-Africa hypothesis (Johnson and Andrews, 2011).
Sadly, what used to be a superpower for improved survival became a disadvantage with devastating health consequences for modern humans. We are much more sensitive to fructose, and therefore sucrose, than other animals. We produce much more uric acid after sugar consumption which has many negative health implications.
Sugar and gout – Why wine did not add greatly to the joy of living
Gout is a modern civilisation disease and has been associated with the consumption of large amounts of meat. But why is that?
Meat, which consists of cells including DNA, is full of purines, such as the nucleic acids adenine (A) and guanine (G). Beer, which has been called the greatest invention in the history of mankind (Dave Barry) contains yeast that is also full of DNA and purines. The metabolism of purines results in the production of uric acid (image 4). As a result, meat and beer can precipitate gout.
However, fructose metabolism that also leads to uric acid production has been shown to be a major risk factor for gout. In the 19th century, gout was described as an illness of the aristocracy. Much more meat was consumed in these circles, but also large amounts of sugar. Great amounts of sugar were added to wines and other beverages. In London, there is a pub called ‘The Crown and Sugar Loaf’ that dates from the early 19th century, and here very sweet drinks were served to its patrons. A painting from this time by George Cruikshank shows an aristocratic and obese man eating fruit and drinking wine, with the demon burning his foot symbolising the pain of gout (image 5) (Rivard et al., 2013).

Image 5: “A self-indulgent man afflicted with gout: the pain is represented by a demon burning his foot” (coloured lithograph by G. Cruikshank, 1818, after Captain Hehl).

Image 5: The front of ‘The Crown and Sugar Loaf’ pub in the present day.
Sugar and high blood pressure – The pantry as a first line of defence (h3)
When it comes to high blood pressure (hypertension), salt has always been one of the main culprits. In order to reduce blood pressure, the dietary intake of salt needs to be reduced; a low salt diet.
After a high salt meal, the salt concentration in the blood rises (serum sodium concentration) and the blood pressure shoots up for a couple of hours. In addition to salt, sugar also plays a role in initiating high blood pressure. Here, several mechanisms such as the promotion of salt and water retention, insulin resistance and nitric oxide deficiency seem to interplay.
When the sodium concentration in the blood is high, the enzymatic conversion of glucose into fructose is induced (via the sorbitol-aldose reductase pathway). The endogenously produced fructose then raises blood pressure. And once again, uric acid plays a central role in this (image 6).
Animal studies showed that the consumption of fructose leads to salt-sensitive hypertension in rats. When allopurinol, a drug to lower uric acid concentration, was administered blood pressure returned to normal (Xu et al., 2017; Eren et al., 2019).
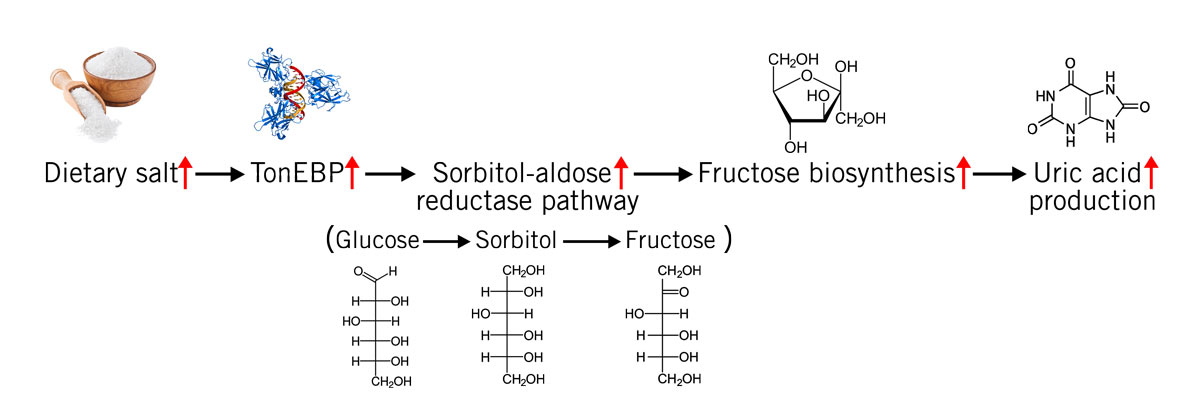
Image 6: Salt consumption activates the transcription factor tonicity-responsive enhancer binding protein (TonEBP), which in turn activates the sorbitol-aldose reductase pathway (conversion of glucose via sorbitol to fructose) that leads to uric acid production via ADP metabolisation (see image 4).
Not satisfied yet? Want to read more about the effects of sugar?
In the next blog post “The evils of sugar – This is where the scientific fun starts“, we will look into the mechanisms of sugar consumption on the cellular level. Get ready for a little bit of biochemistry and refresh your cellular energy production knowledge.
Disclaimer: This text is for general information purposes only and does not constitute the practice of medicine or health care services including the giving of medical advice. The use of this information is at the user’s own risk and is not intended as a substitute for medical advice, diagnosis or treatment.
By Dr Andreas Ebertz
Did you like this article about the effects of sugar on human evolution? Then subscribe to our Newsletter and we will keep you informed about our next blog posts. Subscribe to the Eurofins Genomics Newsletter.







One Comment