Biomarkers are becoming increasingly important for disease diagnostics and treatment. Even though genetic biomarkers are of special significance in disease diagnostics and treatment (diseases and disorders are mainly caused by genetic alterations), protein biomarkers move further towards the centre of attention. After all, almost every clinical treatment of a disease or disorder targets a protein.
Key takeaways:
- Protein biomarkers are proteins, often circulating in the blood stream, that are used to detect diseases and monitor disease progression/treatment response.
- Protein biomarkers have been found for different types of cancer, cardiovascular diseases and neurological disorders.
- Proximity extension assays in combination with NGS, the basis for the Olink technology, enable highly sensitive and specific detection of protein biomarkers.
Protein biomarkers are proteins that can be detected and quantified and provide information about disease and disease progression as well as treatment response and efficacy.
Working with protein biomarkers can prove to be difficult, as often large numbers of samples need to be analysed, especially in research. This is where the Olink technology comes in. It combines proximity extension assays with next-generation sequencing to enable protein biomarker detection with outstanding sensitivity and specificity for high-throughput applications.
Protein biomarkers for cancer
In cancer research, a lot of work has been done to find protein biomarkers circulating in the blood. Protein biomarker panels specific for the detection of colorectal, cervical, ovarian, prostate, lung, and liver cancer have been developed. These proximity extension assay-panels have shown high target sensitivity and reproducibility. For instance, two protein biomarker panels, containing 11 and 27 biomarkers, have been developed that can be used to differentiate between ovarian cancer and a benign tumour (sensitivity: 88%, specificity: 92%, accuracy (AUC): 0.92). Proteins that are tested for include CA125, HE4, Kallikrein-11 and Folate receptor alpha. Interestingly, the panel-based assays outperform ultrasound scans, often the first test when issues with the ovaries are suspected, when done by “ordinary” gynaecologists, not, however, when clinical specialists perform the ultrasound scans.
Similarly, 19 protein biomarkers in blood have been identified that can be used to differentiate between gastric cancer samples and controls with a high diagnostic sensitivity and specificity (sensitivity: 93%, specificity: 100%, accuracy (AUC): 0.99).
The promising results emphasis the huge potential of protein biomarkers for cancer diagnostics and monitoring of disease progression (Enroth et al., 2018 and 2019; Shen et al., 2019; Landegren and Hammond, 2021; Ding et al., 2022; Davies et al., 2023).
Protein biomarkers for cardiovascular disease
In the field of cardiovascular disease research and diagnostics, established protein biomarkers are troponin T and troponin I, among others. Both are found at elevated levels in the blood after cardiac muscle damage caused by myocardial infarction. Recent studies broadened the range of protein biomarkers that show exceptional performance and could be used for diagnosis of myocardial infarction. These protein biomarkers, including IL-6 and ST2, are involved in the inflammatory response and have been found using the Olink technology.
Olink technology has also been used successfully to find protein biomarkers associated with cardiovascular death in patients with coronary heart disease. These protein biomarkers circulating in the blood include brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP), proteins involved in kidney injury (Cys-C), hemodynamic shear stress, and others. It has been proposed that the measurement of the profile of these proteins could inform the treatment of patients with coronary heart disease and even lead to new future targets for treatment (Cao et al., 2019; Mohammad et al, 2020; Wallentin et al., 2021; Wu et al., 2021; Liu et al., 2023; Siegbahn et al., 2023).
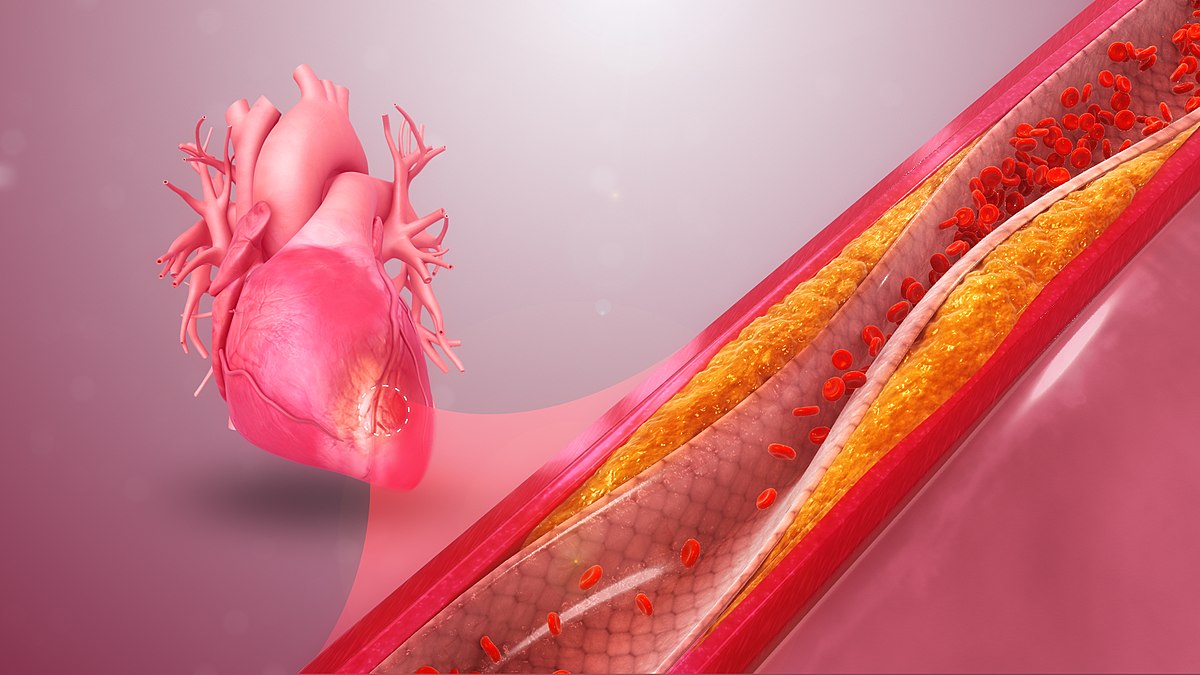
Image 1: Depiction of coronary artery disease. Cholesterol deposits, or plaques buildup in the walls of an artery, reducing the blood, oxygen and nutrient supply to the heart.
Protein biomarkers for neurological disorders
Scientists researching neurological disorders, such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis, have already found protein biomarkers that are used for diagnostics. Most notably are amyloid beta peptide (Aβ), the main component of senile plaques, and tau protein, which accumulates in misfolded form inside neurons, as protein biomarkers for Alzheimer’s disease (AD). However, both protein biomarkers do not seem to correlate well with disease progression.
In a study by Del Campo et al. (2022), the scientist identified and analysed more than 100 proteins in cerebrospinal fluid samples that are dysregulated in AD patients. They created a 12 proteins biomarker panel for proximity extension assay and were able to differentiate between AD, non-AD dementias and controls based on the results. The researchers emphasised the efficiency and effectiveness of proximity extension assay technology to create panels for protein biomarker development. The high degree of customisation of these panels makes them highly interesting for routine diagnostics and clinical trials of drugs against AD. Overall, Olink technology has been shown to be reliable and robust for the simultaneous evaluation of circulating protein biomarkers for AD (Chen et al., 2017; Carlyle et al., 2022).
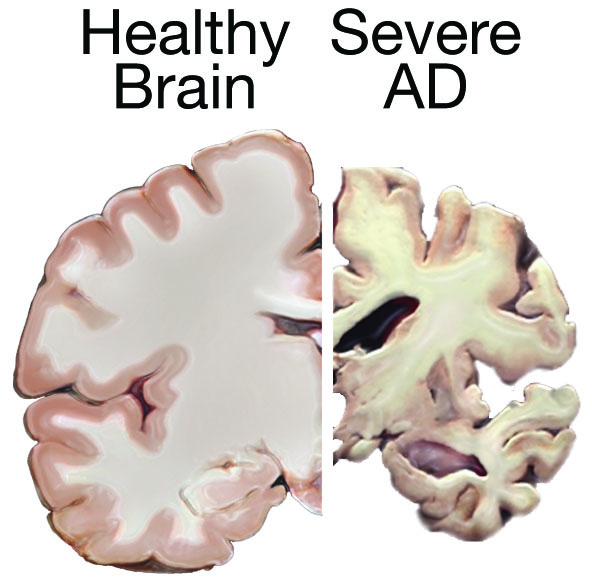
Image 2: Comparison of a healthy brain (left) and brain with severe Alzheimer’s disease (right).
Finding protein biomarkers with Olink technology
In the past, the detection of protein biomarkers via immunoassays was often limited by cross-reactions of antibodies (unspecific binding) in multiplexed assays (pooling of numerous samples). The combination of the proximity extension assay and next-generation sequencing, however, changed this.
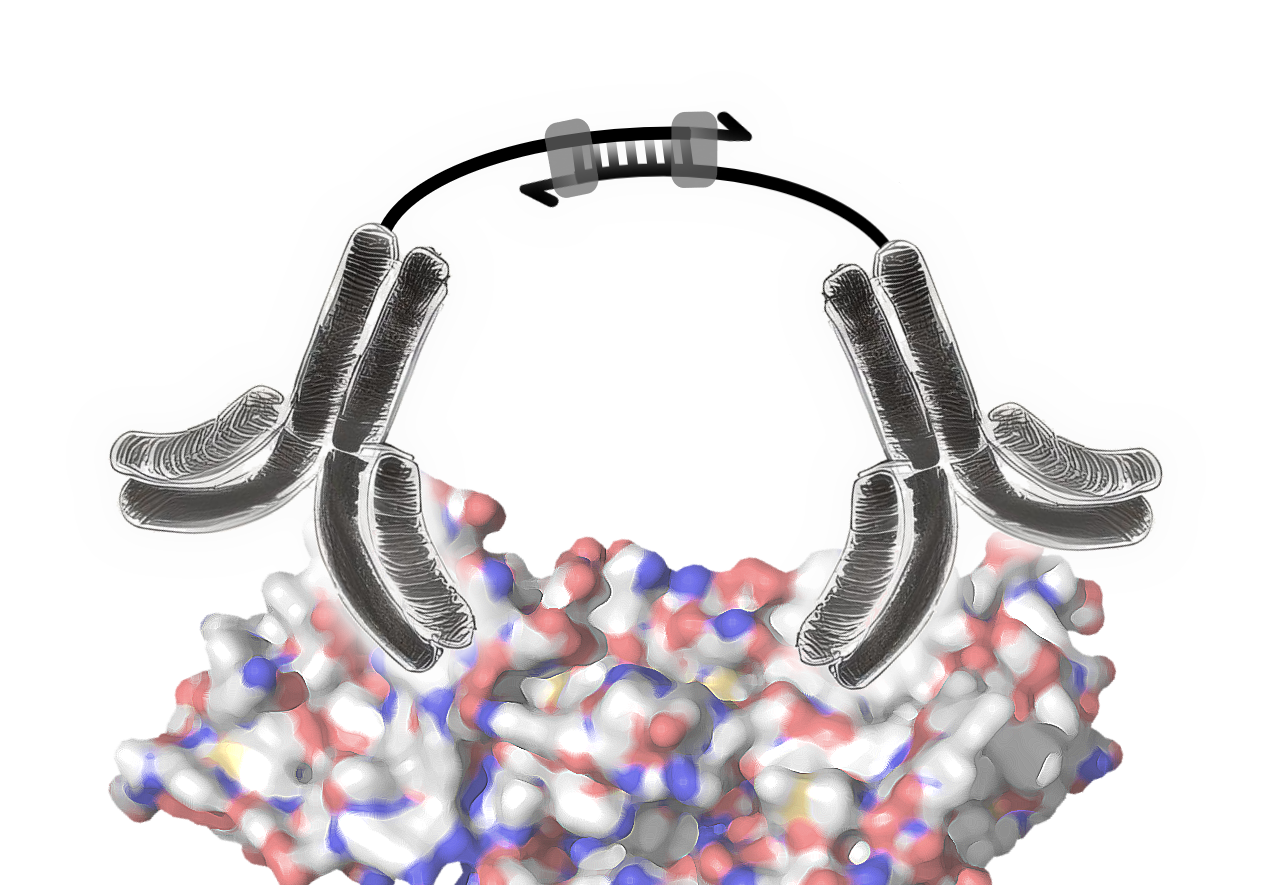
The Olink technology is based on the proximity extension assay that uses pairs of antibodies labelled with oligonucleotides (ssDNA). These pairs of antibodies are highly specific for their target proteins, where they bind in close proximity. When an antibody pair binds to its target protein, the close proximity of both enables a hybridisation of their oligonucleotides. The resulting double stranded DNA sequence then acts as template for DNA polymerase for the synthesis of the complementary DNA strand (image 3).
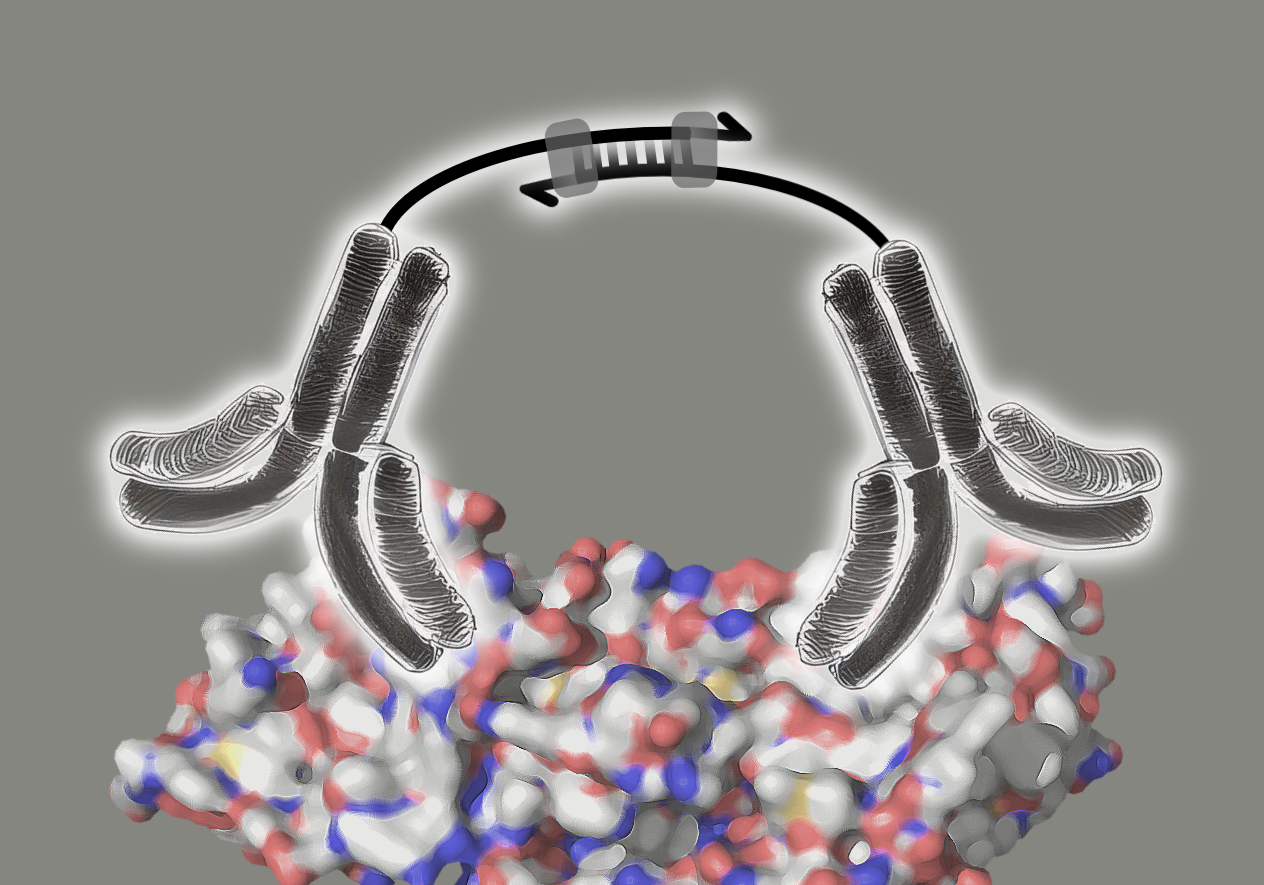
Image 3: Mode of action of proximity extension assay antibodies. The pair of antibodies specifically bind to the target protein. The antibodies’ ssDNA oligonucleotides hybridise, forming a dsDNA sequence. DNA polymerase binds to the dsDNA and extends the ssDNA to dsDNA.
The resulting amplicons can then be detected and quantified using next-generation sequencing. Here, a unique sequence of oligonucleotides attached to the antibodies functions as barcode for the identification of the target protein.
Find out how these barcodes are used for identification using next-generation sequencing.
The big advantage of the Olink technology is the high specificity. It is based on the use of two antibodies that must bind in close proximity on the target protein. Only close proximity allows for the hybridisation of the ssDNA oligonucleotides. Therefore, reducing the occurrence of false positive results due to non-specific binding of antibodies.
The Olink technology also offers high sensitivity. Due to the DNA amplification step, the target protein is amplified, even if it is in low concentration in the original sample. Therefore, a larger range of target proteins can be detected. Additionally, as the amount of amplified DNA is proportional to the amount of target protein in the original sample, a quantification of the targeted protein biomarkers is possible (Wik et al., 2021)
It is safe to say that Olink technology will increasingly contribute to population genetics studies , pre-clinical development and clinical trials, and facilitate our understanding of how proteins affect diseases and disorders.
Eurofins Genomics utilises Olink technology to offer solutions for disease research, drug discovery and drug development research, and more.
Did you like this article about Olink technology? Then subscribe to our Newsletter and we will keep you informed about our next blog posts. Subscribe to the Eurofins Genomics Newsletter.
References:
Cao, Z., Jia, Y., Zhu, B. (2019) BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int J Mol Sci. 20(8):1820.
Carlyle, B.C., Kitchen RR, Mattingly Z, Celia AM, Trombetta BA, Das S, Hyman BT, Kivisäkk P, Arnold SE. (2022) Technical Performance Evaluation of Olink Proximity Extension Assay for Blood-Based Biomarker Discovery in Longitudinal Studies of Alzheimer’s Disease. Front Neurol. 13:889647.
Chen, Gf., Xu, Th., Yan, Y. et al. (2017) Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin. 38: 1205-35.
Davies, M.P.A., Sato, T., Ashoor H, Hou L, Liloglou T, Yang R, Field JK. (2023) Plasma protein biomarkers for early prediction of lung cancer. EBioMedicine. 93:104686.
Del Campo, M., Peeters CFW, Johnson ECB, Vermunt L, Hok-A-Hin YS, van Nee M, Chen-Plotkin A, Irwin DJ, Hu WT, Lah JJ, Seyfried NT, Dammer EB, Herradon G, Meeter LH, van Swieten J, Alcolea D, Lleó A, Levey AI, Lemstra AW, Pijnenburg YAL, Visser PJ, Tijms BM, van der Flier WM, Teunissen CE. (2022) CSF proteome profiling across the Alzheimer’s disease spectrum reflects the multifactorial nature of the disease and identifies specific biomarker panels. Nat Aging, 2(11):1040-53.
Ding, Z., Wang, N., Ji, N. et al. (2022) Proteomics technologies for cancer liquid biopsies. Mol Cancer, 21(1):53.
Enroth, S., Berggrund, M., Lycke M, Lundberg M, Assarsson E, Olovsson M, Stålberg K, Sundfeldt K, Gyllensten U. (2018) A two-step strategy for identification of plasma protein biomarkers for endometrial and ovarian cancer. Clin Proteomics. 15:38.
Enroth, S., Berggrund, M., Lycke M, Broberg J, Lundberg M, Assarsson E, Olovsson M, Stålberg K, Sundfeldt K, Gyllensten U. (2019) High throughput proteomics identifies a high-accuracy 11 plasma protein biomarker signature for ovarian cancer. Commun Biol. 2:221.
Landegren, U. and Hammond, M. (2021) Cancer diagnostics based on plasma protein biomarkers: hard times but great expectations. Mol Oncol. 15(6):1715-26.
Liu, J., Chen, B., Lu, H., Chen, Q., Li, J.C. (2023) Identification of novel candidate biomarkers for acute myocardial infarction by the Olink proteomics platform. Clin Chim Acta. 548:117506.
Mohammad, M.A., Koul, S., Egerstedt A, Smith JG, Noc M, Lang I, Holzer M, Clemmensen P, Gidlöf O, Metzler B, Engstrøm T, Erlinge D. (2020) Using proximity extension proteomics assay to identify biomarkers associated with infarct size and ejection fraction after ST-elevation myocardial infarction. Sci Rep. 10(1):18663.
Shen, Q., Polom, K., Williams, C., de Oliveira FMS, Guergova-Kuras M, Lisacek F, Karlsson NG, Roviello F, Kamali-Moghaddam M. (2019) A targeted proteomics approach reveals a serum protein signature as diagnostic biomarker for resectable gastric cancer. EBioMedicine. 44:322-33.
Siegbahn, A., Eriksson N, Assarsson E, Lundberg M, Ballagi A, Held C, Stewart RAH, White HD, Åberg M, Wallentin L. (2023) Development and validation of a quantitative Proximity Extension Assay instrument with 21 proteins associated with cardiovascular risk (CVD-21). PLoS One, 14;18(11):e0293465.
Wik, L., Nordberg N, Broberg J, Björkesten J, Assarsson E, Henriksson S, Grundberg I, Pettersson E, Westerberg C, Liljeroth E, Falck A, Lundberg M. (2021) Proximity Extension Assay in Combination with Next-Generation Sequencing for High-throughput Proteome-wide Analysis. Mol Cell Proteomics, 20:100168.
Wallentin, L., Eriksson N, Olszowka M, Grammer TB, Hagström E, Held C, Kleber ME, Koenig W, März W, Stewart RAH, White HD, Åberg M, Siegbahn A. (2021) Plasma proteins associated with cardiovascular death in patients with chronic coronary heart disease: A retrospective study. PLoS Med. 18(1):e1003513.
Wu, Y., Nana Pan, Yi An, Mengyuan Xu, Lijuan Tan, Lijuan Zhang (2021) Diagnostic and Prognostic Biomarkers for Myocardial Infarction. Front. Cardiovasc. Med., Sec. General Cardiovascular Medicine, 7: 617277