To stay healthy, it is necessary to keep the melatonin level in the body under control, however more important are prevention and detection of abnormal melatonin concentration in the body.
We, the iGEM Team Aachen 2018, aim to create an efficient and specific melatonin detection system which will be based on yeast cells. This way, we plan to establish a new system to improve current melatonin detection devices and methods. Currently, melatonin is measured with the enzyme-linked immunosorbent assay (ELISA) which uses antibodies. This method is expensive and labor-intensive, making melatonin measurement a costly diagnostic method. Our mission is to provide a faster, cheaper and non-invasive method.
This is how we do it
To get a signal from the melatonin binding, two different signalling pathways are used:
1. Enzyme-Fragment Complementation Assay
(Fukutani et al., 2017)
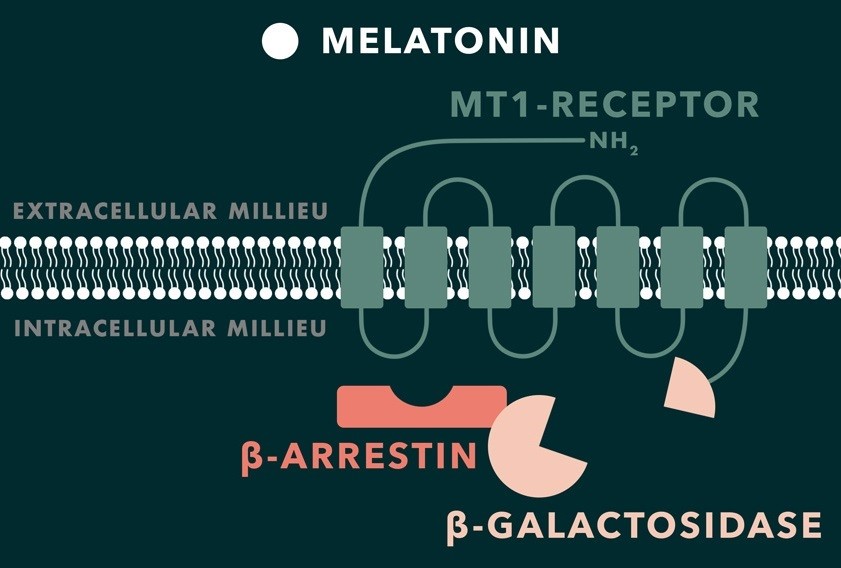
When melatonin binds to the G protein-coupled receptor MT1, β-arrestins are recruited and bind to the receptor. We plan to use this recruitment for an enzyme-fragment complementation assay. On one hand, the MT1 receptor will be fused to a fragment of a reporter enzyme (β -galactosidase from E. coli), whereas, the complementing enzyme fragment will be fused to the β-arrestin. The reporter enzyme is only active and functional if three conditions can be assured: melatonin is present outside the cell, melatonin binds to the receptor surface (MT1) and β-arrestin binds to the cytoplasmic site of the melatonin receptor. If it is the case, it is possible to indirectly measure the concentration of the desired molecules, in this case melatonin, because the two different fragments on MT1 and β-arrestin assemble and form an active enzyme. The substrate (X-gal) interacts with the active enzyme and is transformed to a molecule (5,5′-Dibromo-4,4′-Dichloro-Indigo) with a different colour. Such changes are detectable signs of melatonin presence.
2. Using a nuclear receptor (RZR) and a reporter gene
(Sanseverino et al., 2005)
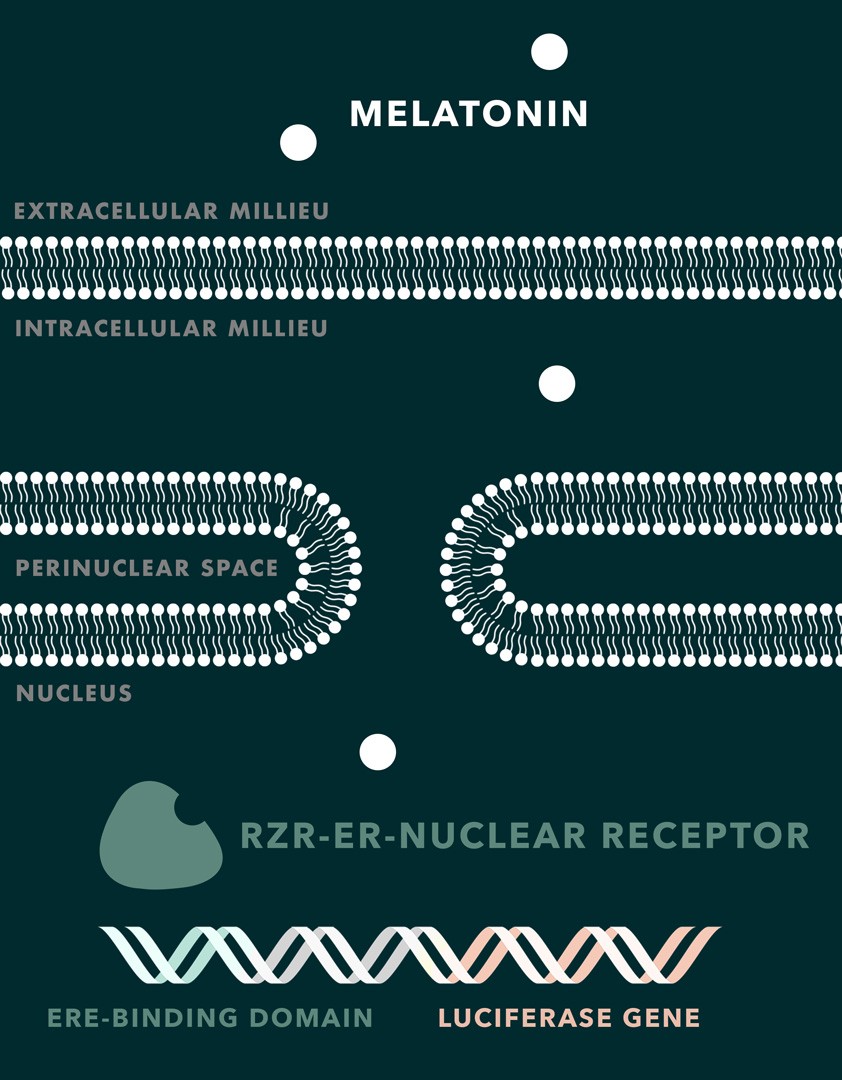
The membrane permeability for melatonin is high and there is theoretically no need for an exogenous receptor to enable the mediation between extracellular melatonin and the yeast cells itself. Thus, the nuclear receptor RZR can be used for melatonin detection. RZR is a human transcription factor that has been shown to upregulate the transcription of certain genes, which help cells dealing with oxidative stress. As the RZR gene does not exist in S. cerevisiae, we are creating a chimeric receptor with a new recognition sequence for human estrogen response element (ERE) and transform it into the yeast cell. There are no published studies about the introduction of RZR in yeast and, therefore, about its functionality and efficiency in yeast cells. Also, it is not known yet if S. cerevisiae has polymerases that are able to interact with the activated RZR receptor and start the transcription of the desired reporter genes. In contrast, ERE is a recognition sequence, which is widely used in S. cerevisiae (Sanseverino et al., 2005). Based on the lack of information, it was decided to substitute the original RZR recognition sequence for the transcription with the recognition sequence of the human estrogen receptor (hER-alpha) fused to a luciferase gene. Activation would lead to the expression of luciferase whose luminescence then can be quantitatively measured.
Melatonin detection in saliva will be based on modified yeast cells. In order to make yeast cells capable of detecting different melatonin concentrations in saliva, it is necessary to incorporate foreign genes from human cells that recreate the melatonin signalling cascade and, additionally, inactivate the yeast genes that are involved in its own melatonin production (Maury et al., 2016).
3. Using localized surface plasmon resonance for a cell-free melatonin measurement
(Kreuzer et al., 2008)
Since the use of microorganisms for the melatonin measurement restricts this diagnostic method to be executed in a laboratory, the development of a cell-free device enables a universal (non-laboratory) usage. By implementing a melatonin-sensitive receptor in a modified yeast cell, we are already able to measure melatonin levels. Because living cells are associated with intensive care, our ultimate goal is to create a cell-free solution that is easy to use. This novel cell-free approach will use a highly sensitive method called localized surface plasmon resonance (LSPR). Here, the binding of a melatonin-sensitive protein to a DNA sequence that is immobilized on a nanoparticle surface affects the refractive index. Smallest differences in refractive index can be detected by a change in optical absorption. Since the change in absorption occurs in the visible wavelength range, we can use a conventional smartphone camera as a sensor.

iGEM Team Aachen 2018
Did you like this article? Then subscribe to our Newsletter and we will keep you informed about our next blog posts. Subscribe to the Eurofins Genomics newsletter.