Stem cells are immature precursor cells with two key abilities — they can reproduce themselves infinitely, and they can differentiate into various specialised cells as needed. Stem cell therapy holds out the promise of novel treatments of disorders, diseases and injuries wherein the body’s own cells have died or stopped functioning.
Key takeaways:
- Stem cell therapy is a fast-growing field in medicine and has great potential for the treatment of many disorders, diseases and injuries.
- Totipotent stem cells can differentiate into any cell type and can form a whole organism.
- Pluripotent stem cells can also differentiate into any cell type, however, cannot form a whole organism.
- Multipotent stem cells can only differentiate into cells types of the tissue they originate from.
- Stem cells are used to treat blood cancers (such as leukaemia), and investigated for the treatment of cardiovascular diseases (heart muscle death) and diabetes (restore pancreatic function).
- Mesenchymal stem cells, in particular, are investigated for cancer treatment and used to restore joint and bone-related diseases and injuries.
The basis of stem cell therapy
The most capable stem cells are the totipotent stem cells found in the zygote. These cells can divide and differentiate to form different tissues, complete organs, and the placenta and umbilical cord.
The stem cells of clinical interest were classified into embryonic (pluripotent) and adult (multipotent) types.
Pluripotent stem cells have the valuable ability to differentiate into all cell types in the body and are usually only found in embryos. Recently, however, scientists have been able to convert adult cells into pluripotent cells by introducing certain genes encoding for transcription factors using retroviruses (Takahashi, 2007). These cells, called induced pluripotent stem cells (iPSCs), have vital advantages for the application in disease and injury treatment – since they are obtained from the person being treated, there is no need for immunosuppression treatment (autologous transplant), whereas the use of embryonic stem cells often requires immunosuppression (allogeneic transplant). The use of iPSCs also sidestep the ethical concerns associated with using embryonic stem cells that originate from human embryos.
Multipotent stem cells are partially specialised and can only differentiate into the cells of the organ where they originate from. For example, skin stem cells can differentiate into dermal cells, epidermal cells and fibroblasts. Although limited in their differentiation abilities, multipotent stem cells are valuable in maintaining tissues by replacing damaged or aging cells. They are found in foetuses, umbilical cord blood, and the placenta, so the term ‘adult stem cells’ is a misnomer (Ishiuchi et al., 2015; Tabansky and Stern, 2016).

Depiction of cell potency with totipotent, pluripotent and multipotent cells.
The possibilities and limits of stem cell therapy and research
The science of directing and controlling the differentiation of stem cells is still in its infancy. Human stem cell research would require obtaining cells from 5- to 7-day old blastocysts, destroying the embryo. Since the embryo has the potential to grow into a human being, research using embryonic pluripotent cells is considered unethical. Pluripotent cells also have rarely been used therapeutically in humans.
In animal studies on pluripotent cells, a major danger was the formation of solid tumours called teratomas.
In contrast, multipotent stem cells are under intense research, specifically how differentiation is affected by chemical as well as physical stimulation, and how it can be controlled. Careful combinations of growth factors, activins, and other chemicals, applied together and sequentially, are being investigated. The aim is to induce stem cells to differentiate and multiply toward a specific, needed lineage. A potential application is the direction of marrow stem cells to form erythrocytes in people with red blood cell formation disorders. Human pluripotent stem cells are also being investigated for the treatment of diabetes. Differentiated into pancreatic beta cells, they can secrete insulin in response to a high blood glucose level, reversing diabetes (Lo and Parham, 2009; The International Stem Cell Initiative, 2018; Memon and Abdelalim, 2020).

How are stem cells harvested for stem cell therapy
Stem cells are mainly harvested from:
- Blood – stem cells in the blood are filtered out and the remaining blood is returned to the body
- Bone marrow – bone marrow of the hip bone is extracted with a needle and syringe and the stem cells are extracted
- Cord blood – stem cells in the blood are filtered out.
Stem cells are then either used directly (intravenous, soft tissue or joint Injection), or further differentiated into specific cells before application (Babic and Trigoso, 2019, NHS, 2022).
Stem cell therapy in clinical applications
At present, a surprisingly small number of stem cell therapies are accepted for human clinical use. The best established therapeutic use of stem cells is in the treatment of blood cancers such as leukaemia, lymphoma and multiple myeloma. More than a million procedures have been performed in the last sixty years, making this the most successful application of stem cell therapy (Cieri et al., 2021).
Apart from cancers, stem cell therapy is also useful to treat and manage disorders of blood cell formation such as Fanconi’s anaemia, a rare disease characterised by bone marrow failure. Here, stem cell therapy is utilised with high success rates (Ebens et al., 2017).
Skin, bone and corneal grafting are not considered stem cell therapy per se, however, the success of the grafts depend on stem cells within the transplanted tissue.
Future applications of stem cell therapy for cardiovascular diseases
The leading causes of death worldwide are cardiovascular diseases (more than 18.5 million deaths in 2019). Here, coronary artery disease is responsible for around 16 % of the world’s total deaths. Patients who suffer from coronary artery disease for example are at risk of developing heart failure that often results from the lack of functional myocytes (heart muscle cells). Thus, the most obvious application of stem cell therapy is in the field of cardiovascular diseases.
Stem cell therapy is being investigated for the treatment of myocyte death and expected to create new, functional heart tissue. Stem cells also release cardioprotective paracrine factors that activate endogenous pathways, leading to myocardial repair. Animal studies and sporadic reports in humans have shown significant benefits in heart disease, but transplanted myocytes do not engraft well. At present, this promising stem cell application is not yet close to clinical use (Nguyen 2016; Ritchie et al., 2018; WHO, 2020).
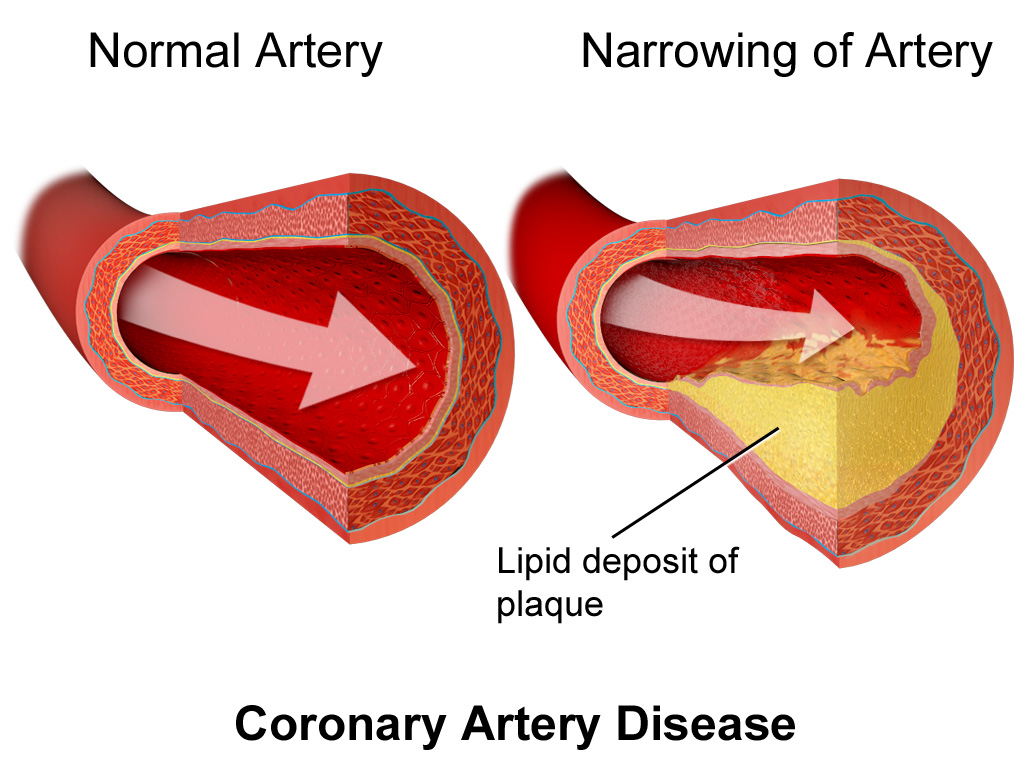
Depiction of coronary artery disease, where plaques builds up in the arteries of the heart, leading to reduced blood flow to the heart muscle and myocyte death
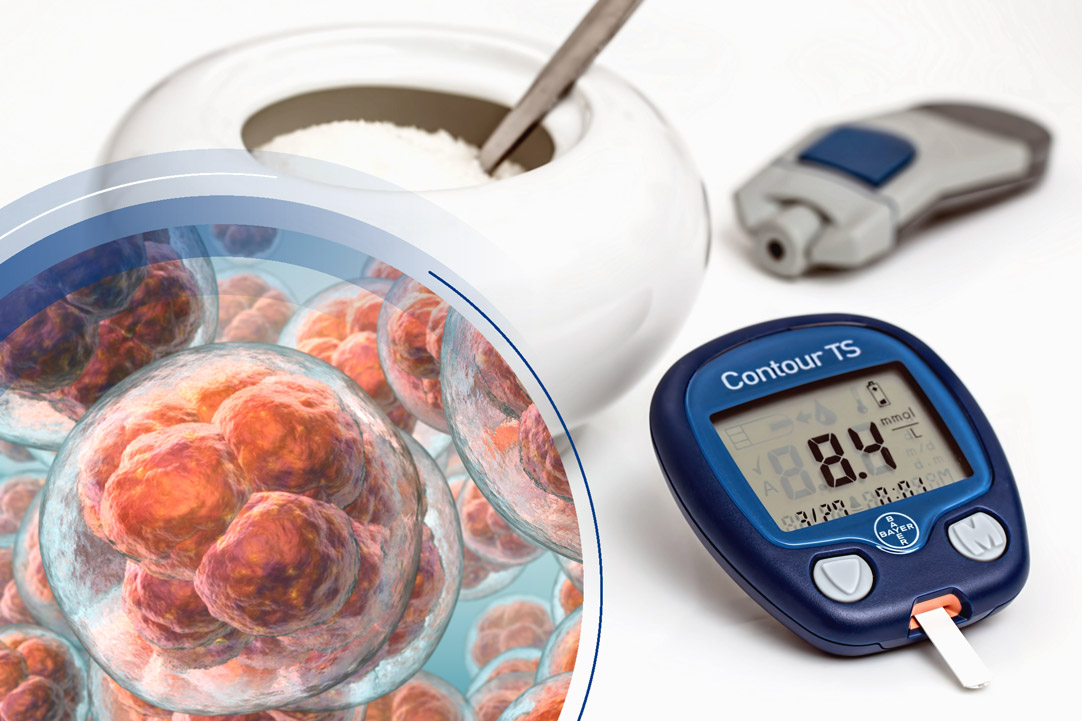
Future applications of stem cell therapy for diabetes
Diabetes mellitus type 1 results from a lack of insulin production by the pancreas. Injectable insulin is the current standard treatment, but it is demanding and unsatisfactory, as the injection of exogenous insulin is done in fixed doses once or several times a day, potentially leading to both hyperglycemia and hypoglycemic episodes.
Various protocols are attempting to differentiate pluripotent stem cells into insulin-producing cells (β-cells). Transplanted into the pancreas, they could restore the normal pancreatic function and provide the precise control of blood glucose that exogenous insulin cannot (Memon and Abdelalim, 2020).
Future applications of stem cell therapy for neurological disorders
Stem cell therapy holds much promise for the treatment of several neurological disorders (affecting the brain, spinal cord and nerves throughout the body) to restore neuronal function. These disorders include diabetic neuropathy, trigeminal neuralgia, stroke, amyotrophic lateral sclerosis (ALS), age-related macular degeneration, Parkinson’s disease, and multiple sclerosis.
An exciting potential use of stem cells is in spinal cord injuries. The hope is that they can repair injured nerves and remyelinate them. There has been some success in animal studies, but more knowledge about synapse formation, axon invasion, chemo-attractants, and other matters is needed. The research into this stem cell application is promising and neural circuits restoration and functional recovery may be achieved in the future (Shinozaki et al., 2021).
Mesenchymal stem cells for future applications of stem cell therapy
Mesenchymal stem cells (MSCs), which are multipotent stem cells, are a great hope in cancer treatment. By themselves, they inhibit tumour vascularisation, slow cancer growth, and retard epithelial-mesenchymal transition in some cancers (epithelial cells become mesenchymal stem cells that can migrate and invade).
Since MSCs can migrate to tumour sites, they are attractive as vehicles for precisely-delivered antitumor drugs, oncolytic viruses (can kill tumour cells directly or indirectly), and “suicide genes”. However, caution is needed, as MSCs promote mitosis and play a role in the initiation, development, progression, and metastasis of cancer. Nevertheless, the potential of MSCs in cancer therapy seems enormous and more research is needed to utilise their benefits without enhancing the malignancy (Lan et al., 2021).
MSCs can also be used to treat degenerative changes in joints, reconstruct bones and cartilage, and are likely to become standard treatment for osteoarthritis. Such stem cell therapy is also being tried for neuropathic pain, musculoskeletal pain, intervertebral disc disease, restoration of facial expressions and regaining of movements in paralysed patients. Other interesting features of MSCs are their ability to suppress the immune response and act immunomodulatory and antimicrobial (Musiał-Wysocka, et al.; 2019).

Stem cell therapy is enormously exciting but not yet clinically applicable for the vast majority of uses. Currently, stem cell therapy for human treatment is approved for only a few disorders, chiefly using blood-forming (hematopoietic) progenitor cells. Though more research is needed, stem cell therapy will soon fulfil its potential and alleviate much human suffering.
This may also be of interest to you:
High resolution cancer diagnostics with next-generation sequencing
By Dr. Parang Mehta and Dr. Andreas Ebertz
Did you like this article about stem cell therapy? Then subscribe to our Newsletter and we will keep you informed about our next blog posts. Subscribe to the Eurofins Genomics Newsletter.