
Image 1: Axolotl and starfish

Stem cell therapy – A matter of the heart
Dave Randle, a 49-year-old bus driver, had a heart attack in March 2016. He suffered from heart failure with dyspnea (breathing difficulties due to fluid build-up in the lungs) and had diminished abilities to exert any effort, stating that he “could barely walk upstairs”. Due to his refusal of surgical treatment, his physician suggested stem cell therapy. This involved stem cells being infused into his heart to restore the function of the damaged tissues. Within weeks of the treatment, Mr. Randle began to feel better and went back to performing everyday activities.
Stem cell therapy – The beginning
Let’s first rewind a little bit to the origins of stem cell research and stem cell therapy.
In 1958 the French oncologist, Georges Mathé, performed the first stem cell transplantation of bone marrow grafts to save six nuclear researchers who were accidentally exposed to radiation.
In the early 1960s, Ernest McCulloch and James Till (a cellular biologist and a biophysicist respectively at the University of Toronto) discovered haematopoietic stem cells (HSCs) and demonstrated their role in blood cell formation through a series of experiments in mice. A few years later, HSCs were shown to possess the ability to self-renew, one of the defining features of stem cells (figure 2).
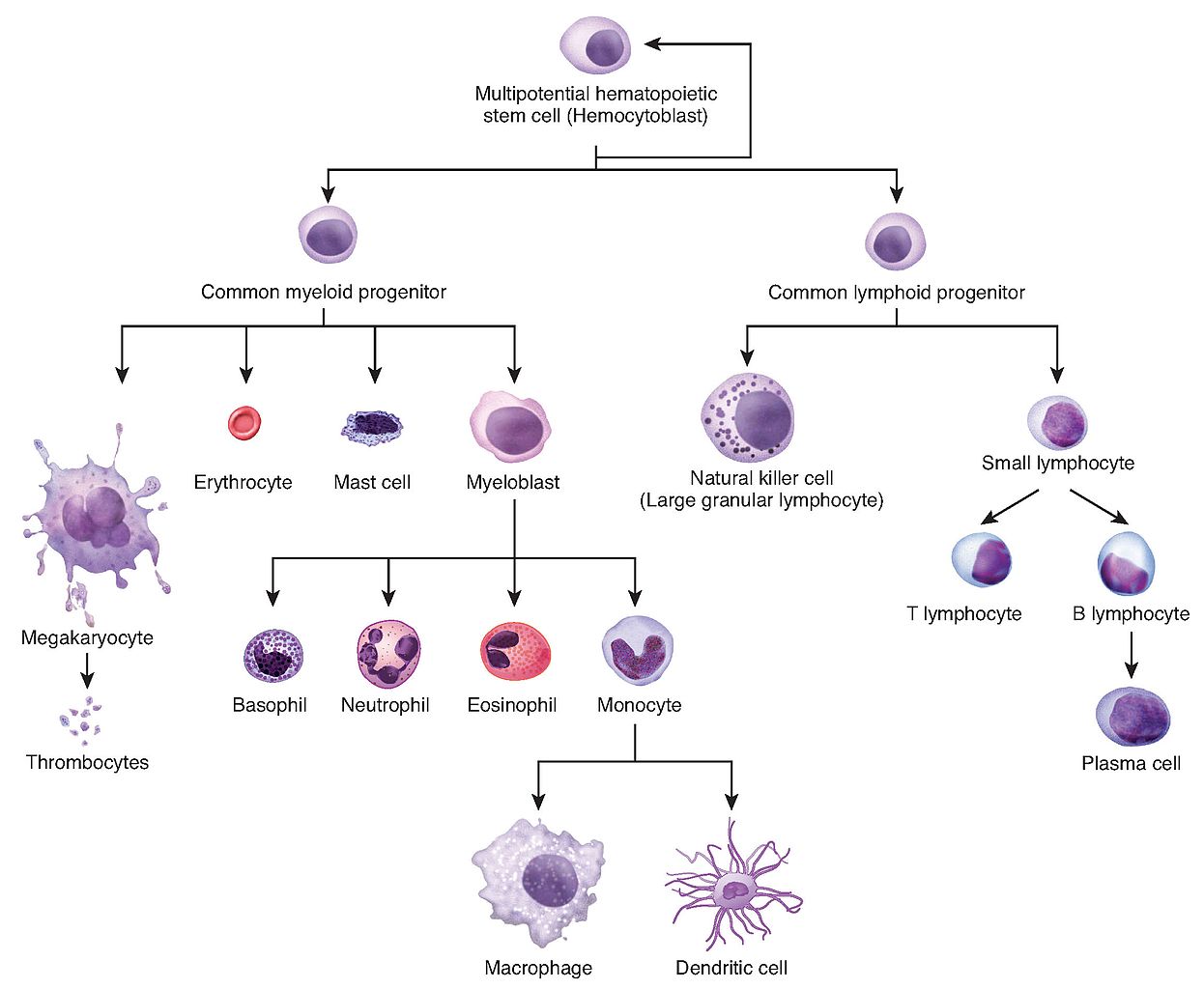
Figure 2: Depiction of haematopoiesis.
After the success of his first bone marrow transplant therapy, Georges Marthé once again astonished the scientific community by curing a case of leukaemia using bone marrow transplantation in 1963 (figure 3).

Figure 3: Georges Mathé, Ernest McCulloch (Photo courtesy of the University Health Network).
In order to delve deeper into the science behind stem cells, we will examine the types of stem cells and their characteristics.
Embryonic stem cells
Embryonic stem cells, formed in the blastocyst and, have not undergone any stage of differentiation yet. They are pluripotent, meaning they can give rise to any type of cell in the body (image 4).
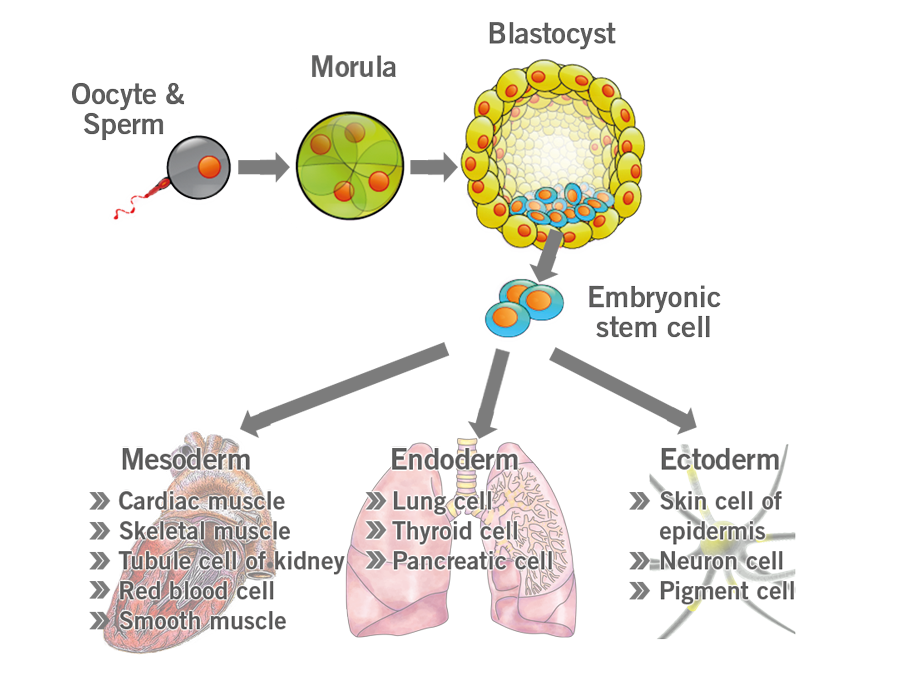
Image 4: Depiction of embryonic stem cell differentiation.
Adult stem cells
Adult stem cells are more specialised than embryonic stem cells and can be found in different organs throughout the body, where they divide and further differentiate to replenish lost cells.
Unlike embryonic stem cells, they are multipotent, meaning they can only differentiate into specific cell types. For instance, neural stem cells can only differentiate into glial and neuronal cells.
Mesenchymal stem cells (mesenchymal stromal cells) are another type of adult stem cells that have been featured prominently in clinical trials recently due to their ability to differentiate into a variety of cell types. They can be induced to differentiate into neuronal precursors and mature neurons, a promising development in the treatment of central nervous system disease. Additionally, they can modulate the innate immune response and provide trophic support. Trophic factors modulate the local immune system, enhance angiogenesis, prevent cell apoptosis, and stimulate the survival, proliferation, and differentiation of resident tissue stem cells.
Induced pluripotent stem cells (iPSCs)
Somatic cells can be converted into stem cells through the introduction of specific genes (e.g. Myc, Oct3/4, Sox2 and Klf4) that encode transcription factors (Yamanaka factors) for the conversion of somatic cells into pluripotent stem cells.
Those factors were discovered by Dr. Shinya Yamanaka in 2006 (hence the name Yamanaka factors), and were found to:
- Erase the identity of a cell (de-specialisation);
- Revert the cell back to its embryonic state.
Recently, it has been discovered that exposing cells to a lower dose of Yamanaka factors for a shorter period of time has an age reversal effect without the subsequent loss of cell identity, which opened up the door to a whole new avenue of longevity and anti-ageing research.
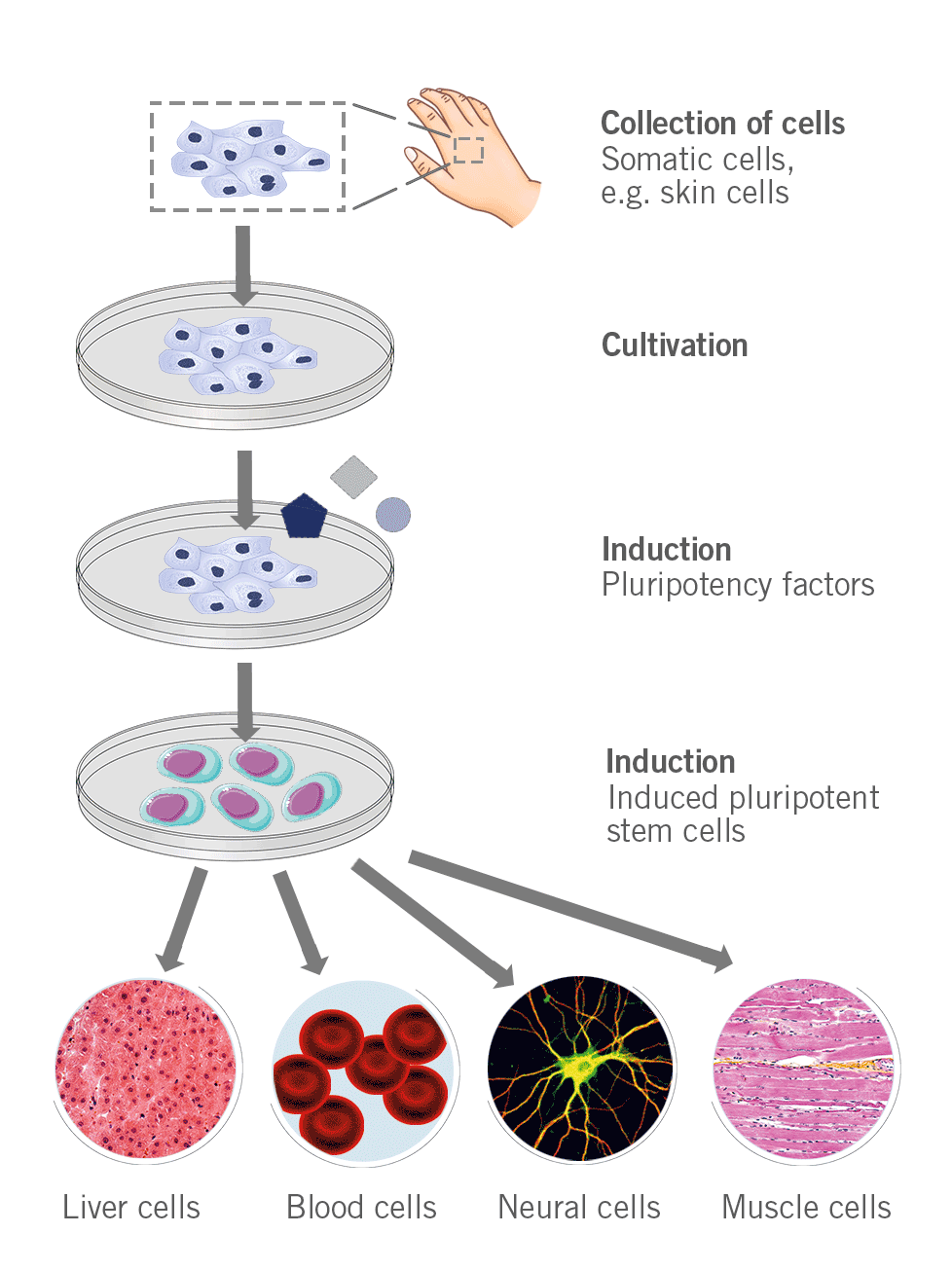
Figure 5: Depiction of the process to generate induced pluripotent stem cells.
iPSCs have the following advantages over embryonic stem cells:
- No risk of immune system reaction or rejection, as iPSCs carry the patient’s own genetic material;
- Avoiding the controversy of whether using embryos for experimental therapeutic purposes is ethical or not.
An important concept in stem cell therapy involves the fate of stem cells, which is explained below.
- A common fate of adult multipotent stem cells is quiescence, where they neither differentiate nor divide e.g. haemopoietic cells waiting for stimuli from the body.
- Stem cells don’t differentiate, but rather divide to give rise to daughter cells that are exactly identical to the parent cell. These daughter cells then contribute to the stem cell pool. This is called symmetric self-renewal.
- Stem cells divide into two cells, one of which is identical to the parent stem cell, while the other is a more differentiated cell, called a progenitor. This is called asymmetric self-renewal.
- Stem cells divide to give rise to two more differentiated daughter cells with loss in the stem cell pool. This is called symmetric division without self-renewal (figure 6).
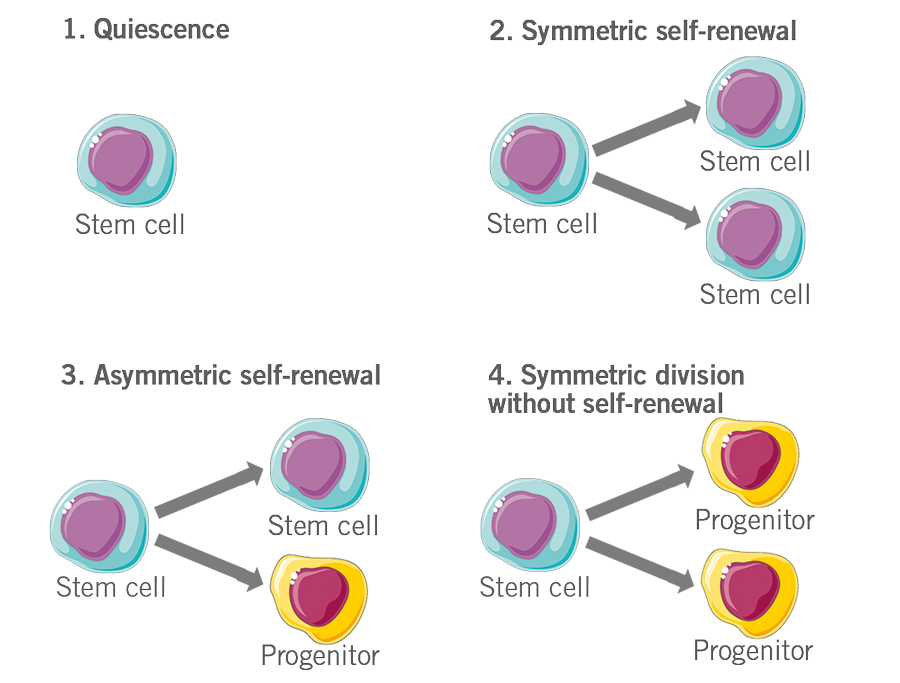
Figure 6: Depiction of the fate of stem cells.
The journey of stem cell therapy, which started with a bone marrow transplant over 60 years ago, has flourished throughout the years to become an essential therapeutic method for many incurable diseases (e.g. multiple sclerosis).
Longevity-associated genes, mechanisms of tumourigenesis, the essence of regenerative medicine, and various other new fields of intriguing research were uncovered as a result of studying different types and fates of stem cells.
Let us know what you think about this article and leave a comment below!
By Abd-ElRahman Samhan and Dr Andreas Ebertz
Did you like this article on stem cell therapy? Then subscribe to our Newsletter and we will keep you informed about our next blog posts. Subscribe to the Eurofins Genomics Newsletter.





2 Comments